Biology:Tus
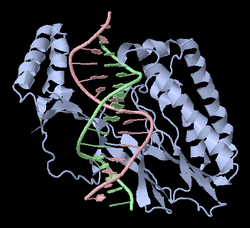
Tus, also known as terminus utilization substance, is a protein that binds to terminator sequences and acts as a counter-helicase when it comes in contact with an advancing helicase.[2] The bound Tus protein effectively halts DNA polymerase movement.[2] Tus helps end DNA replication in prokaryotes.[2]
In E. coli, Tus binds to 10 closely related sites encoded in the chromosome. These sites bind 23 base-pairs. The 10 sites are called Ter sites, and are designated TerA, TerB, ..., TerJ. These binding sites are asymmetric, such that when a Tus-Ter complex (Tus protein bound to a Ter site) is encountered by a replication fork from one direction, the complex is dissociated and replication continues (permissive). But when encountered from the other direction, the Tus-Ter complex provides a much larger kinetic barrier and halts replication (non-permissive). The multiple Ter sites in the chromosome are oriented such that the two oppositely moving replication forks are both stalled in the desired termination region.[3]
Bacillus subtilis utilize replication terminator protein (RTP) instead of Tus.
Protein domains
The Ter protein contains two domains. The N-terminal domain is composed of an alpha helices, beta sandwich, and three loops. The C-terminal domain is made of two alpha helices and one beta sheet.[4]
Further reading
- "Interaction of the Escherichia coli replication terminator protein (Tus) with DNA: a model derived from DNA-binding studies of mutant proteins by surface plasmon resonance."[5]
- "Replication termination in Escherichia coli: structure and antihelicase activity of the Tus-Ter complex."[6]
- "A molecular mousetrap determines polarity of termination of DNA replication in E. coli."[3]
- "Isolation and characterization of mutants of Tus, the replication arrest protein of Escherichia coli." [7]
- "Biophysical characteristics of Tus, the replication arrest protein of Escherichia coli."[8]
- "Structure of a replication-terminator protein complexed with DNA."[1]
References
- ↑ 1.0 1.1 Kamada, K.; Horiuchi, T.; Ohsumi, K.; Shimamoto, N.; Morikawa, K. (1996). "Structure of a replication-terminator protein complexed with DNA". Nature 383 (6601): 598–603. doi:10.1038/383598a0. PMID 8857533. Bibcode: 1996Natur.383..598K.
- ↑ 2.0 2.1 2.2 Slonczewski, Joan, and John Watkins. Foster. Microbiology: An Evolving Science. New York: W.W. Norton &, 2009. Print.
- ↑ 3.0 3.1 Mulcair, M.; Schaeffer, P.; Oakley, A.; Cross, H.; Neylon, C.; Hill, T.; Dixon, N. (2006). "A Molecular Mousetrap Determines Polarity of Termination of DNA Replication in E. Coli". Cell 125 (7): 1309–1319. doi:10.1016/j.cell.2006.04.040. PMID 16814717.
- ↑ "Structure of a replication-terminator protein complexed with DNA.". Nature 383 (6601): 598–603. 1996. doi:10.1038/383598a0. PMID 8857533. https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8857533.
- ↑ Neylon; Brown, S. E.; Kralicek, A. V.; Miles, C. S.; Love, C. A.; Dixon, N. E. (2000). "Interaction of the Escherichia coli replication terminator protein (Tus) with DNA: a model derived from DNA-binding studies of mutant proteins by surface plasmon resonance". Biochemistry 39 (39): 11989–11999. doi:10.1021/bi001174w. PMID 11009613. https://eprints.soton.ac.uk/26769/1/0012-BiochemTus.pdf.
- ↑ Neylon, C.; Kralicek, A. V.; Hill, T. M.; Dixon, N. E. (2005). "Replication Termination in Escherichia coli: Structure and Antihelicase Activity of the Tus-Ter Complex". Microbiology and Molecular Biology Reviews 69 (3): 501–26. doi:10.1128/MMBR.69.3.501-526.2005. PMID 16148308.
- ↑ Skokotas; Wrobleski, M.; Hill, T. M. (1994). "Isolation and characterization of mutants of Tus, the replication arrest protein of Escherichia coli". The Journal of Biological Chemistry 269 (32): 20446–20455. PMID 8051142.
- ↑ Coskun-ari; Skokotas, A.; Moe, G. R.; Hill, T. M. (1994). "Biophysical characteristics of Tus, the replication arrest protein of Escherichia coli". The Journal of Biological Chemistry 269 (6): 4027–4034. PMID 8307958.
