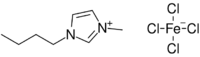
Chemistry:1-Butyl-3-methylimidazolium tetrachloroferrate
From HandWiki
Short description: Magnetic liquid
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| Properties | |
| C8H15Cl4FeN2 | |
| Molar mass | 336.87 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
1-Butyl-3-methylimidazolium tetrachloroferrate is a magnetic ionic liquid. It can be obtained from 1-butyl-3-methylimidazolium chloride and ferric chloride. It has quite low water solubility.[1][2]
Due to the presence of the high spin FeCl4 anion, the liquid is paramagnetic and a magnetic susceptibility of 40.6 × 10−6 emu g−1 is reported. A simple small neodymium magnet suffices to attract the liquid in a test tube.
References
- ↑ Satoshi Hayashi; Hiro-o Hamaguchi (2004). "Discovery of a Magnetic Ionic Liquid [bmim]FeCl4". Chemistry Letters 33 (18): 1590–1591. doi:10.1246/cl.2004.1590.
- ↑ Satoshi Hayashi; Satyen Saha; Hiro-o Hamaguchi (2006). "A new class of magnetic fluids: bmim[FeCl4] and nbmim[FeCl4] ionic liquids". IEEE Transactions on Magnetics 42 (1): 12–14. doi:10.1109/TMAG.2005.854875. Bibcode: 2006ITM....42...12H.
category:ferrates
 |

