Chemistry:1-Methylcytosine
From HandWiki
Short description: Chemical compound
| |
| Names | |
|---|---|
| Preferred IUPAC name
4-Amino-1-methylpyrimidin-2(1H)-one | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| MeSH | 1-Methylcytosine |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C5H7N3O | |
| Molar mass | 125.131 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
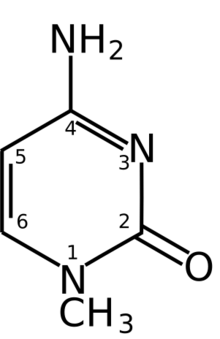
1-Methylcytosine is a methylated form of the DNA base cytosine.
In 1-methylcytosine, a methyl group is attached to the 1st atom in the 6-atom ring. This methyl group distinguishes 1-methylcytosine from cytosine.
History
Miriam Rossi worked on the refinement of 1-methylcytosine.[1]
1-Methylcytosine is used as a nucleobase of hachimoji DNA, in which it pairs with isoguanine.[2]
References
- ↑ Kistenmacher, T. J.; Rossi, M. (1977-12-15). "1-Methylcytosine: a refinement". Acta Crystallographica Section B 33 (12): 3962–3965. doi:10.1107/S0567740877012618. ISSN 0567-7408. Bibcode: 1977AcCrB..33.3962R.
- ↑ Hoshika, Shuichi (22 February 2019). "Hachimoji DNA and RNA: A genetic system with eight building blocks". Science 363 (6429): 884–887. doi:10.1126/science.aat0971. PMID 30792304. Bibcode: 2019Sci...363..884H.
 |

