Chemistry:1-Methyltryptophan
| |
| Names | |
|---|---|
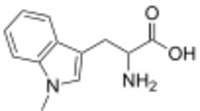
| Preferred IUPAC name
2-Amino-3-(1-methyl-1H-indol-3-yl)propanoic acid | |
| Other names
1-Methyl-DL-tryptophan; DL-1-Methyltryptophan; ARBRIN; Indoximod
| |
| Identifiers | |
| |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C12H14N2O2 | |
| Molar mass | 218.256 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
1-Methyltryptophan is a chemical compound that is an inhibitor of the tryptophan catabolic enzyme indoleamine 2,3-dioxygenase (IDO or INDO EC 1.13.11.52).[1] It is a chiral compound that can exist as both D- and L-enantiomers.
The L-isomer (L-1MT) inhibits IDO weakly but also serves as an enzyme substrate.
The D-isomer (D-1MT) does not inhibit IDO at all, but it can inhibit the IDO-related enzyme IDO2[2] and restore mTOR signaling in cells starved of tryptophan due to IDO activity.[3] D-1MT is also known as indoximod and is currently in clinical trials for cancer treatment, such as for advanced melanoma.[4]
A U.S. patent covering salt and prodrug formulations of indoximod was issued to NewLink Genetics on August 15, 2017 providing exclusivity until at least 2036.[5][6]
References
- ↑ Cady, SG; Sono, M (1991). "1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-3-benzo[b]thienyl-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase". Archives of Biochemistry and Biophysics 291 (2): 326–33. doi:10.1016/0003-9861(91)90142-6. PMID 1952947.
- ↑ Metz, R; Duhadaway, J. B.; Kamasani, U; Laury-Kleintop, L; Muller, A. J.; Prendergast, G. C. (2007). "Novel tryptophan catabolic enzyme IDO2 is the preferred biochemical target of the antitumor indoleamine 2,3-dioxygenase inhibitory compound D-1-methyl-tryptophan". Cancer Research 67 (15): 7082–7. doi:10.1158/0008-5472.CAN-07-1872. PMID 17671174.
- ↑ Metz, R; Rust, S; Duhadaway, J. B.; Mautino, M. R.; Munn, D. H.; Vahanian, N. N.; Link, C. J.; Prendergast, G. C. (2012). "IDO inhibits a tryptophan sufficiency signal that stimulates mTOR: A novel IDO effector pathway targeted by D-1-methyl-tryptophan". OncoImmunology 1 (9): 1460–1468. doi:10.4161/onci.21716. PMID 23264892.
- ↑ IDO Inhibitors Emerging as New Players on Checkpoint Blockade Scene. Aug 2017
- ↑ "NewLink Genetics". https://finpedia.co/bin/Companies/NewLink%20Genetics%20Corp/.
- ↑ "NewLink Genetics Annual Report (Form 10-K)". April 9, 2018. https://fintel.io/doc/sec-nlnk-newlink-genetics-10ka-2018-april-09-17956.
 |

