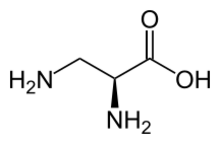
Chemistry:2,3-Diaminopropionic acid
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
3-Amino-L-alanine
| |
| Systematic IUPAC name
(2S)-2,3-Diaminopropanoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C3H8N2O2 | |
| Molar mass | 104.109 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
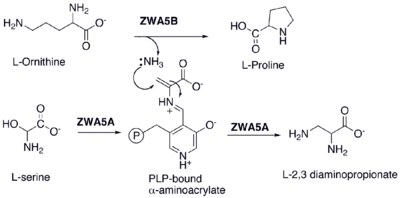
2,3-Diaminopropionic acid (2,3-diaminopropionate, Dpr)[1] is a non-proteinogenic amino acid found in certain secondary metabolites, including zwittermicin A[2] and tuberactinomycin.[3]
Biosynthesis
2,3-Diaminopropionate is formed by the pyridoxal phosphate (PLP) mediated amination of serine.
References
- ↑ "Selective incorporation of proteinaceous over nonproteinaceous cationic amino acids in model prebiotic oligomerization reactions". Proceedings of the National Academy of Sciences of the United States of America 116 (33): 16338–16346. August 2019. doi:10.1073/pnas.1904849116. PMID 31358633.
- ↑ "Asymmetric synthesis of diastereomeric diaminoheptanetetraols. A proposal for the configuration of (+)-zwittermicin a". Organic Letters 9 (3): 437–40. February 2007. doi:10.1021/ol062804a. PMID 17249781.
- ↑ "Deciphering tuberactinomycin biosynthesis: isolation, sequencing, and annotation of the viomycin biosynthetic gene cluster". Antimicrobial Agents and Chemotherapy 47 (9): 2823–30. September 2003. doi:10.1128/AAC.47.9.2823-2830.2003. PMID 12936980.