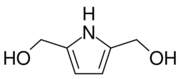
Chemistry:2,5-Bis(hydroxymethyl)pyrrole
| |
| Names | |
|---|---|
| Preferred IUPAC name
(1H-Pyrrole-2,5-diyl)dimethanol | |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H9NO2 | |
| Molar mass | 127.143 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,5-Bis(hydroxymethyl)pyrrole is an organic chemical compound with formula C
6H
9O
2N, or (HOCH
2)
2(C
4H
3N). Its molecule can be described as that of pyrrole C
4H
5N with hydroxymethyl groups HO–CH
2– replacing the two hydrogen atoms adjacent to the nitrogen atom.
The compound is a white solid, soluble in water and acetone. It is stable in alkaline solutions, but otherwise tends to polymerize by self-condensation.[1] The compound was used as an intermediate in the synthesis of hexahydroporphine ("unsubstituted porphyrinogen"),[1] the core of uroporphyrinogen III, precursor of many critically important biological products such as hemoglobin and chlorophyll.
Preparation
The compound can be synthesized by formylation of pyrrole followed by reduction of the resulting pyrrolecarboxaldehyde.[1]
References
- ↑ 1.0 1.1 1.2 Taniguchi, Shozo; Hasegawa, Hikaru; Yanagiya, Shoko; Tabeta, Yusuke; Nakano, Yoshiharu; Takahashi, Masahiko (2001). "The first isolation of unsubstituted porphyrinogen and unsubstituted 21-oxaporphyrinogen by the '3+1' approach from 2,5-bis(hydroxymethyl)pyrrole and tripyrrane derivatives". Tetrahedron 57 (11): 2103–2108. doi:10.1016/S0040-4020(01)00059-X.
 |

