Chemistry:2,5-Diamino-6-hydroxy-4-(5-phosphoribosylamino)pyrimidine
From HandWiki
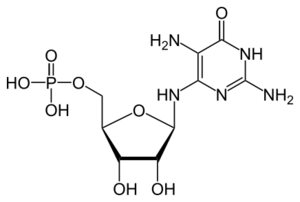
| |
| Names | |
|---|---|
| IUPAC name
(1R)-1,4-Anhydro-1-[(2,5-diamino-6-oxo-1,6-dihydropyrimidin-4-yl)amino]-D-ribitol 5-(dihydrogen phosphate)
| |
| Systematic IUPAC name
{(2R,3S,4R,5R)-5-[(2,5-Diamino-6-oxo-1,6-dihydropyrimidin-4-yl)amino]-3,4-dihydroxyoxolan-2-yl}methyl dihydrogen phosphate | |
| Other names
2,5-Diamino-6-ribofuranosylamino-4(3H)-pyrimidinone monophosphate; N-(2,5-Diamino-6-hydroxypyrimidin-4-yl)-5-O-phosphono-β-D-ribofuranosylamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI |
|
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C9H16N5O8P | |
| Molar mass | 353.23 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
2,5-diamino-6-hydroxy-4-(5-phosphoribosylamino)pyrimidine is a metabolite in the purine metabolism, formed by the hydrolysis of GTP by GTP cyclohydrolase II.[1] Alternatively two separate enzymes can carry out this reaction, initially GTP cyclohydrolase IIa hydrolyses the 8,9 bond to form 2-Amino-5-formylamino-6-(5-phospho-D-ribosylamino)pyrimidin-4(3H)-one,[2] followed by de-formylation by 2-amino-5-formylamino-6-ribosylaminopyrimidin-4(3H)-one 5'-monophosphate deformylase.[3] 2,5-diamino-6-hydroxy-4-(5-phosphoribosylamino)pyrimidine is deaminated by Diaminohydroxyphosphoribosylaminopyrimidine deaminase to form 5-amino-6-(5-phosphoribosylamino)uracil.[4]
References
- ↑ "Purification and properties of guanosine triphosphate cyclohydrolase II from Escherichia coli". J. Biol. Chem. 250 (9): 3545–51. 1975. doi:10.1016/S0021-9258(19)41549-4. PMID 235552.
- ↑ "A member of a new class of GTP cyclohydrolases produces formylaminopyrimidine nucleotide monophosphates". Biochemistry 41 (50): 15074–84. 2002. doi:10.1021/bi0268798. PMID 12475257.
- ↑ Grochowski, L.L.; Xu, H.; White, R.H. (2009). "An iron(II) dependent formamide hydrolase catalyzes the second step in the archaeal biosynthetic pathway to riboflavin and 7,8-didemethyl-8-hydroxy-5-deazariboflavin". Biochemistry 48 (19): 4181–4188. doi:10.1021/bi802341p. PMID 19309161.
- ↑ "Presence of Escherichia coli of a deaminase and a reductase involved in biosynthesis of riboflavin". J. Bacteriol. 136 (2): 657–67. 1978. doi:10.1128/JB.136.2.657-667.1978. PMID 30756.
 |

