Chemistry:3'-Hydroxyechinenone
From HandWiki
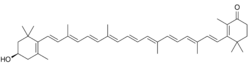
| |
| Names | |
|---|---|
| IUPAC name
(3′R)-3′-Hydroxy-β,β-caroten-4-one
| |
| Systematic IUPAC name
3-{(1E,3E,5E,7E,9E,11E,13E,15E,17E)-18-[(4R)-4-Hydroxy-2,6,6-trimethylcyclohex-1-en-1-yl]-3,7,12,16-tetramethyloctadeca-1,3,5,7,9,11,13,15,17-nonen-1-yl}-2,4,4-trimethylcyclohex-2-en-1-one | |
| Other names
LMPR01070098; 3'-OH-Echinenone; C15965
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C40H54O2 | |
| Molar mass | 566.870 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
3′-Hydroxyechinenone is a keto-carotenoid pigment found in cyanobacteria and microalgae.[1] Carotenoids belong to a larger class of phytochemicals known as terpenoids. The chemical formula of canthaxanthin is C40H54O2. It is found non-covalently bound in the orange carotenoid protein (OCP), which is a soluble protein involved in photoprotection and non-photochemical quenching of photosynthesis.[2]
References
- ↑ Grung, Merete; Metzger, Pierre; Liaaen-jensen, Synnove (1989). "Primary and secondary carotenoids in two races of the green alga Botryococcus braunii". Biochemical Systematics and Ecology 17 (4): 263–269. doi:10.1016/0305-1978(89)90001-x.
- ↑ Kirilovsky, Diana; Kerfeld, Cheryl A. (2013). "The orange carotenoid protein: A blue-green light photoactive protein". Photochemical & Photobiological Sciences 12 (7): 1135–43. doi:10.1039/c3pp25406b. ISSN 1474-905X. PMID 23396391.
 |

