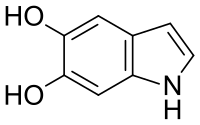
Chemistry:5,6-Dihydroxyindole
| |
| Names | |
|---|---|
| IUPAC name
1H-Indole-5,6-diol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C8H7NO2 | |
| Molar mass | 149.149 g·mol−1 |
| Hazards | |
| GHS pictograms |   
|
| GHS Signal word | Danger |
| H302, H318, H411 | |
| P264, P264+265Script error: No such module "Preview warning".Category:GHS errors, P270, P273, P280, P301+317Script error: No such module "Preview warning".Category:GHS errors, P305+354+338Script error: No such module "Preview warning".Category:GHS errors, P317Script error: No such module "Preview warning".Category:GHS errors, P330, P391, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5,6-Dihydroxyindole is a chemical compound with the molecular formula C8H7NO2. It is an intermediate in the production of the biological pigment eumelanin.[2] 5,6-Dihydroxyindole is biosynthesized from L-dopachrome in a reaction catalyzed by a tyrosinase enzyme and is further converted into indole-5,6-quinone.[3] In humans, 5,6-dihydroxyindole is involved in the metabolic disorder hawkinsinuria.[3]
In some insects, 5,6-dihydroxyindole is a reactive compound that is produced as a component of defense responses and has antibacterial and antifungal activity.[4]
A laboratory synthesis of 5,6-dihydroxyindole can be accomplished starting from 3,4-dibenzyloxybenzaldehyde.[5] This compound is condensed with nitromethane in a Henry reaction, followed by nitration, reduction of the nitro groups, and hydrogenolysis of the benzyl protecting groups.
References
- ↑ "5,6-Dihydroxyindole" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/114683#section=Safety-and-Hazards.
- ↑ d'Ischia, Marco; Napolitano, Alessandra; Pezzella, Alessandro (2011). "5,6-Dihydroxyindole Chemistry: Unexplored Opportunities Beyond Eumelanin". European Journal of Organic Chemistry 2011 (28): 5501–5516. doi:10.1002/ejoc.201100796.
- ↑ 3.0 3.1 "5,6-Dihydroxyindole". Human Metabolome Database. https://hmdb.ca/metabolites/HMDB0004058.
- ↑ Zhao, Picheng; Lu, Zhiqiang; Strand, Michael R.; Jiang, Haobo (2011). "Antiviral, anti-parasitic, and cytotoxic effects of 5,6-dihydroxyindole (DHI), a reactive compound generated by phenoloxidase during insect immune response". Insect Biochemistry and Molecular Biology 41 (9): 645–652. doi:10.1016/j.ibmb.2011.04.006. PMID 21554953.
- ↑ Crane, Stuart W.; Ghafur, Omair; Cowie, Thomas Y.; Lindsay, Anita G.; Thompson, James O. F.; Greenwood, Jason B.; Bebbington, Magnus W. P.; Townsend, Dave (2019). "Dynamics of electronically excited states in the eumelanin building block 5,6-dihydroxyindole". Physical Chemistry Chemical Physics 21 (15): 8152–8160. doi:10.1039/C9CP00620F. PMID 30933211. https://pureadmin.qub.ac.uk/ws/files/195373535/56_DHI_AAM.pdf.
 |

