Chemistry:5Beta-Scymnol
From HandWiki
Short description: Synthetic skin conditioning ingredient
| |
| Names | |
|---|---|
| IUPAC name
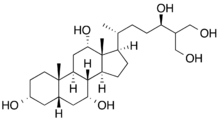
(3R,5S,7R,8R,9S,10S,12S,13R,14S,17R)-17-[(2R,5R)-5,7-Dihydroxy-6-(hydroxymethyl)heptan-2-yl]-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-3,7,12-triol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C27H48O6 | |
| Molar mass | 468.675 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5Beta-Scymnol, also known simply as scymnol, is a synthetic INCI-listed skin conditioning ingredient.[1][2][3] The molecule is a steroid derivative that behaves as a hydroxyl radical scavenger and is used for the treatment of skin blemishes such as blocked pores and acne.[4][5]
History
The molecule was identified and isolated from shark tissues by Professor Takuo Kosuge, Shizuoka College of Pharmacy, Shizuoka, Japan during the 1980s.[4] Based on usage as a traditional folk remedy, it was hypothesised the ingredient may be effective for the treatment of scalds, blemishes and acne.[6][7][8]
Traits
5Beta-Scymnol is a hydroxyl (OH) free radical scavenger.[5] Scymnol's role in quenching free radicals may play a role in inhibiting acne.[8][9]
References
- ↑ "INCI". https://www.personalcarecouncil.org/resources/inci/.
- ↑ "SODIUM SCYMNOL SULFATE - Ingrédient INCI Beauty". https://incibeauty.com/ingredients/7746-sodium-scymnol-sulfate.
- ↑ Dunlop, K.J.; Barnetson, R.S. (February 1995). "A comparative study of isolutrol versus benzoyl peroxide in the treatment of acne". The Australasian Journal of Dermatology 36 (1): 13–15. doi:10.1111/j.1440-0960.1995.tb00917.x. ISSN 0004-8380. PMID 7763215. https://pubmed.ncbi.nlm.nih.gov/7763215/.
- ↑ 4.0 4.1 Chaston, Ian (2017-01-28) (in en). Technological Entrepreneurship: Technology-Driven vs Market-Driven Innovation. Springer. ISBN 978-3-319-45850-2. https://books.google.com/books?id=IfcODgAAQBAJ&dq=%22scymnol%22+%22takuo+kosuge%22&pg=PA251.
- ↑ 5.0 5.1 Macrides et al, A comparison of the hydroxyl radical scavenging properties of the shark bile steroid 5β-scymnol and plant pycnogenols, Biochem Mol Biol Int. 1997 Sep;42(6):1249-60
- ↑ Kosuge, Y., Kosuge, T., Tsuji, K., Ishida, H., and Broadbent, J. M. (1989). Scymnol sulphate salts isolated from shark tissues for the treatment of liver and skin ailments (Patent: PCTInt Appl. WO 8801274-C.I. CO7J31/ 00). Chem. Abslr. 110, 88640g
- ↑ Muthusamy, Visalini; Hodges, Lynn D.; Macrides, Theodore A.; Boyle, Glen M.; Piva, Terrence J. (2011). "Effect of Novel Marine Nutraceuticals on IL-1α-Mediated TNF-α Release from UVB-Irradiated Human Melanocyte-Derived Cells". Oxidative Medicine and Cellular Longevity 2011: 728645. doi:10.1155/2011/728645. ISSN 1942-0900. PMID 21961050.
- ↑ 8.0 8.1 Guardiola-Griffiths, Cristina (2011-09-01). "Medieval mean girls: on sexual rivalry and the uses of cosmetics in La Celestina" (in English). EHumanista 19: 172–193. https://go.gale.com/ps/i.do?p=AONE&sw=w&issn=15405877&v=2.1&it=r&id=GALE%7CA360475533&sid=googleScholar&linkaccess=abs.
- ↑ Zasloff, M.; Adams, A. P.; Beckerman, B.; Campbell, A.; Han, Z.; Luijten, E.; Meza, I.; Julander, J. et al. (2011). "Squalamine as a broad-spectrum systemic antiviral agent with therapeutic potential". Proceedings of the National Academy of Sciences of the United States of America 108 (38): 15978–15983. doi:10.1073/pnas.1108558108. PMID 21930925. Bibcode: 2011PNAS..10815978Z.
