Chemistry:5β-Scymnol
| |
| Names | |
|---|---|
| IUPAC name
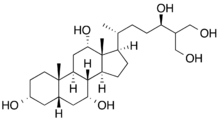
(3R,5S,7R,8R,9S,10S,12S,13R,14S,17R)-17-[(2R,5R)-5,7-Dihydroxy-6-(hydroxymethyl)heptan-2-yl]-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-3,7,12-triol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C27H48O6 | |
| Molar mass | 468.675 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
5β-Scymnol, also known simply as scymnol, is a synthetic INCI-listed skin conditioning ingredient.[1][2][3] The molecule is a steroid derivative that behaves as a hydroxyl radical scavenger and is used for the treatment of skin blemishes such as blocked pores and acne.[4][5]
History
The molecule was identified and isolated from shark tissues by Professor Takuo Kosuge, Shizuoka College of Pharmacy, Shizuoka, Japan during the 1980s.[4] Based on usage as a traditional folk remedy, it was hypothesised the ingredient may be effective for the treatment of scalds, blemishes and acne.[6][7][8]
Clinical testing
Following Kosuge’s discovery, a sulfated form of scymnol was clinically evaluated as treatment for hyperseborrhea, a condition that occurs when the sebaceous glands produce excess oil in the skin. In 1988, Pierre Fabre conducted a double-blind, placebo controlled clinical trial of a scymnol-based lotion for hyperseborrhea on 40 subjects which was published in La Gazette Medicale. The control group showed no change in skin oiliness but the scymnol group had a near 40% reduction.[9]
Given scymnol’s properties and the relationship between seborrhoea, skin oiliness and acne,[10] the molecule was further investigated for the treatment of acne. David Fenton of St Thomas’ Hospital, London trialed a formulation containing scymnol sulfate over two months. 14 of the 15 participants showed improvement in their acne condition over the course of the trial.[11]
A comparative study of sodium scymnol sulfate versus benzoyl peroxide was published in 1995. Sodium scymnol sulfate significantly improved patients’ acne by reducing the number of inflamed lesions. It did not significantly reduce the numbers of non-inflamed lesions. Fewer side effects were experienced by patients treated with Sodium Scymnol Sulfate than those treated with benzoyl peroxide. The study concluded scymnol was a useful adjunct in the treatment of acne, particularly in patients with inflamed lesions.[12][13][14]
Traits
5β-Scymnol is a hydroxyl (OH) free radical scavenger.[5] Scymnol's role in quenching free radicals may play a role in inhibiting acne.[15][16]
References
- ↑ "INCI". https://www.personalcarecouncil.org/resources/inci/.
- ↑ "SODIUM SCYMNOL SULFATE - Ingrédient INCI Beauty". https://incibeauty.com/ingredients/7746-sodium-scymnol-sulfate.
- ↑ Dunlop, K.J.; Barnetson, R.S. (February 1995). "A comparative study of isolutrol versus benzoyl peroxide in the treatment of acne". The Australasian Journal of Dermatology 36 (1): 13–15. doi:10.1111/j.1440-0960.1995.tb00917.x. ISSN 0004-8380. PMID 7763215. https://pubmed.ncbi.nlm.nih.gov/7763215/.
- ↑ 4.0 4.1 Chaston, Ian (2017-01-28) (in en). Technological Entrepreneurship: Technology-Driven vs Market-Driven Innovation. Springer. ISBN 978-3-319-45850-2. https://books.google.com/books?id=IfcODgAAQBAJ&dq=%22scymnol%22+%22takuo+kosuge%22&pg=PA251.
- ↑ 5.0 5.1 Macrides et al, A comparison of the hydroxyl radical scavenging properties of the shark bile steroid 5β-scymnol and plant pycnogenols, Biochem Mol Biol Int. 1997 Sep;42(6):1249-60
- ↑ Kosuge, Y., Kosuge, T., Tsuji, K., Ishida, H., and Broadbent, J. M. (1989). Scymnol sulphate salts isolated from shark tissues for the treatment of liver and skin ailments (Patent: PCTInt Appl. WO 8801274-C.I. CO7J31/ 00). Chem. Abslr. 110, 88640g
- ↑ Muthusamy, Visalini; Hodges, Lynn D.; Macrides, Theodore A.; Boyle, Glen M.; Piva, Terrence J. (2011). "Effect of Novel Marine Nutraceuticals on IL-1α-Mediated TNF-α Release from UVB-Irradiated Human Melanocyte-Derived Cells". Oxidative Medicine and Cellular Longevity 2011: 728645. doi:10.1155/2011/728645. ISSN 1942-0900. PMID 21961050.
- ↑ Guardiola-Griffiths, Cristina (2011-09-01). "Medieval mean girls: on sexual rivalry and the uses of cosmetics in La Celestina" (in English). EHumanista 19: 172–193. https://go.gale.com/ps/i.do?p=AONE&sw=w&issn=15405877&v=2.1&it=r&id=GALE%7CA360475533&sid=googleScholar&linkaccess=abs.
- ↑ Fabre, P. “Etude contrôlée, en double aveugle contre placebo, d'une lotion à base d'isolutrol dans le traitement de l'hyperséborrhée”, Gazette médicale de France No. 36, 27 October 1989
- ↑ Burton JL, Sbuster S. Tbe relationship between seborrboea and acne vulgaris. Br. J. Dermatol. 1971; 84: 600-1.
- ↑ Isolutrol: A new agent in acne” D. Fenton, Guest Speaker: Australasian College of Dermatologists Annual Meeting (1992) Perth
- ↑ Muthusamy, Visalini; Hodges, Lynn D.; Macrides, Theodore A.; Boyle, Glen M.; Piva, Terrence J. (2011). "Effect of Novel Marine Nutraceuticals on IL-1α-Mediated TNF-α Release from UVB-Irradiated Human Melanocyte-Derived Cells". Oxidative Medicine and Cellular Longevity 2011: 728645. doi:10.1155/2011/728645. ISSN 1942-0900. PMID 21961050.
- ↑ Welch M, Caughman A, Verdicchio RJ, et al. Addressing the role of free radical oxidation in the acne paradigm. Presented at: The 67th Annual Academy of Dermatology Meeting; March 6-10, 2009; San Francisco, California
- ↑ Mills et al, Addressing Free Radical Oxidation in Acne Vulgaris, J Clin Aesthet Dermatol. 2016 Jan; 9(1): 25–30
- ↑ Guardiola-Griffiths, Cristina (2011-09-01). "Medieval mean girls: on sexual rivalry and the uses of cosmetics in La Celestina" (in English). EHumanista 19: 172–193. https://go.gale.com/ps/i.do?p=AONE&sw=w&issn=15405877&v=2.1&it=r&id=GALE%7CA360475533&sid=googleScholar&linkaccess=abs.
- ↑ Zasloff, M.; Adams, A. P.; Beckerman, B.; Campbell, A.; Han, Z.; Luijten, E.; Meza, I.; Julander, J. et al. (2011). "Squalamine as a broad-spectrum systemic antiviral agent with therapeutic potential". Proceedings of the National Academy of Sciences of the United States of America 108 (38): 15978–15983. doi:10.1073/pnas.1108558108. PMID 21930925. Bibcode: 2011PNAS..10815978Z.

