Chemistry:Benzoyl peroxide
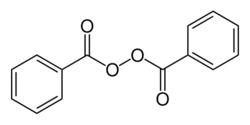 | |
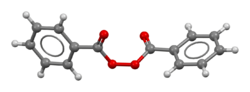 Skeletal formula (top) Ball-and-stick model (bottom) | |
| Clinical data | |
|---|---|
| Trade names | Benzac, Panoxyl, others |
| Other names | benzoperoxide, dibenzoyl peroxide (DBPO), BPO |
| AHFS/Drugs.com | Professional Drug Facts |
| MedlinePlus | a601026 |
| License data | |
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H10O4 |
| Molar mass | 242.230 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.334 g/cm3 |
| Melting point | 103 to 105 °C (217 to 221 °F) decomposes |
| Solubility in water | poor mg/mL (20 °C) |
| |
| |
Benzoyl peroxide is a chemical compound (specifically, an organic peroxide) with the structural formula (C
6H
5–C(=O)O–)
2, often abbreviated as (BzO)2. In terms of its structure, the molecule can be described as two benzoyl (C
6H
5–C(=O)–, Bz) groups connected by a peroxide (–O–O–). It is a white granular solid with a faint odour of benzaldehyde, poorly soluble in water but soluble in acetone, ethanol, and many other organic solvents. Benzoyl peroxide is an oxidizer, which is principally used in the production of polymers.[5]
Benzoyl peroxide is mainly used in production of plastics[5][6] and for bleaching flour, hair, plastics and textiles.[7][8]
As a bleach, it has been used as a medication and a water disinfectant.[6][8]
As a medication, benzoyl peroxide is mostly used to treat acne, either alone or in combination with other treatments.[9] Some versions are sold mixed with antibiotics such as clindamycin.[10][11] It is on the World Health Organization's List of Essential Medicines.[12] It is available as an over-the-counter and generic medication.[13][10] It is also used in dentistry for teeth whitening. In 2021, it was the 284th most commonly prescribed medication in the United States, with more than 700,000 prescriptions.[14][15]
History
Benzoyl peroxide was first prepared and described by Justus von Liebig in 1858.[16] Donald Holroyde Hey FRS[17] (12 September 1904 – 21 January 1987) was a Welsh organic chemist who inferred that the decomposition of benzoyl peroxide generated free phenyl radicals.[18][19]
Structure and reactivity

The original 1858 synthesis by Liebig reacted benzoyl chloride with barium peroxide,[16] a reaction that probably follows this equation:
- 2 C6H5C(O)Cl + BaO2 → (C6H5CO)2O2 + BaCl2
Benzoyl peroxide is usually prepared by treating hydrogen peroxide with benzoyl chloride under alkaline conditions.
- 2 C6H5COCl + H2O2 + 2 NaOH → (C6H5CO)2O2 + 2 NaCl + 2 H2O
The oxygen–oxygen bond in peroxides is weak. Thus, benzoyl peroxide readily undergoes homolysis (symmetrical fission), forming free radicals:
- (C6H5CO)2O2 → 2 C6H5CO•2
The symbol • indicates that the products are radicals; i.e., they contain at least one unpaired electron. Such species are highly reactive. The homolysis is usually induced by heating. The half-life of benzoyl peroxide is one hour at 92 °C. At 131 °C, the half-life is one minute.[21]
In 1901, it was observed that the compound made the tincture of guaiacum tincture turn blue, a sign of oxygen being released.[22] Around 1905, Loevenhart reported on the successful use of benzoyl peroxide to treat various skin conditions, including burns, chronic varicose leg tumors, and tinea sycosis. He also reported animal experiments that showed the relatively low toxicity of the compound.[23][7][24]
Treatment with benzoyl peroxide was proposed for wounds in 1929, and for sycosis vulgaris and acne varioliformis in 1934.[24] However, preparations were often of questionable quality.[7] It was officially approved for the treatment of acne in the US in 1960.[7]
Polymerization
Benzoyl peroxide is mainly used as a radical initiator to induce chain-growth polymerization reactions,[5] such as for polyester and poly(methyl methacrylate) (PMMA) resins and dental cements and restoratives.[25] It is the most important among the various organic peroxides used for this purpose, a relatively safe alternative to the much more hazardous methyl ethyl ketone peroxide.[26][27] It is also used in rubber curing and as a finishing agent for some acetate yarns.[25]
Other uses
Benzoyl peroxide is effective for treating acne lesions. It does not induce antibiotic resistance.[28][29] It may be combined with salicylic acid, sulfur, erythromycin or clindamycin (antibiotics), or adapalene (a synthetic retinoid). Two common combination drugs include benzoyl peroxide/clindamycin and adapalene/benzoyl peroxide, adapalene being a chemically stable retinoid that can be combined with benzoyl peroxide[30] unlike tezarotene and tretinoin. Combination products such as benzoyl peroxide/clindamycin and benzoyl peroxide/salicylic acid appear to be slightly more effective than benzoyl peroxide alone for the treatment of acne lesions.[29] The combination tretinoin/benzoyl peroxide was approved for medical use in the United States in 2021.
Benzoyl peroxide for acne treatment is typically applied to the affected areas in gel, cream, or liquid, in concentrations of 2.5% increasing through 5.0%, and up to 10%.[28] No strong evidence supports the idea that higher concentrations of benzoyl peroxide are more effective than lower concentrations.[28]
Mechanism of action
Classically, benzoyl peroxide is thought to have a three-fold activity in treating acne. It is sebostatic, comedolytic, and inhibits growth of Cutibacterium acnes, the main bacterium associated with acne.[28][31] In general, acne vulgaris is a hormone-mediated inflammation of sebaceous glands and hair follicles. Hormone changes cause an increase in keratin and sebum production, leading to blocked drainage. C. acnes has many lytic enzymes that break down the proteins and lipids in the sebum, leading to an inflammatory response. The free-radical reaction of benzoyl peroxide can break down the keratin, therefore unblocking the drainage of sebum (comedolytic). It can cause nonspecific peroxidation of C. acnes, making it bactericidal,[7] and it was thought to decrease sebum production, but disagreement exists within the literature on this.[31][32]
Some evidence suggests that benzoyl peroxide has an anti-inflammatory effect as well. In micromolar concentrations it prevents neutrophils from releasing reactive oxygen species, part of the inflammatory response in acne.[32]
Side effects

Application of benzoyl peroxide to the skin may result in redness, burning, and irritation. This side effect is dose-dependent.[9][13]
Because of these possible side effects, it is recommended to start with a low concentration and build up as appropriate, as the skin gradually develops tolerance to the irritation caused by the medication. Skin sensitivity typically resolves after a few weeks of continuous use.[32][33] Irritation can also be reduced by avoiding harsh facial cleansers and wearing sunscreen prior to sun exposure.[33]
One in 500 people experience hypersensitivity to benzoyl peroxide and are liable to experience burning, itching, crusting, and possibly swelling.[34][35] About one-third of people experience phototoxicity under exposure to ultraviolet (UVB) light.[36]
Dosage
In the US, the typical concentration for benzoyl peroxide is 2.5% to 10% for both prescription and over-the-counter drug preparations that are used in treatment for acne.[37]
Other medical uses
Benzoyl peroxide is used in dentistry as a tooth whitening product.[38]
Safety
Explosion hazard
Benzoyl peroxide is potentially explosive[39] like other organic peroxides, and can cause fires without external ignition. The hazard is acute for the pure material, so the compound is generally used as a solution or a paste. For example, cosmetics contain only a small percentage of benzoyl peroxide and pose no explosion risk.
Toxicity
Benzoyl peroxide breaks down in contact with skin, producing benzoic acid and oxygen, neither of which is very toxic.[40]
The carcinogenic potential of benzoyl peroxide has been investigated. A 1981 study published in the journal Science found that although benzoyl peroxide is not a carcinogen, it does promote cell growth when applied to an initiated tumor. The study concluded, "caution should be recommended in the use of this and other free radical-generating compounds".[41]
A 1999 IARC review of carcinogenicity studies found no convincing evidence linking benzoyl peroxide acne medication to skin cancers in humans. However, some animal studies found that the compound could act as a carcinogen and enhance the effect of known carcinogens.[25]
Benzoyl peroxide can break down into carcinogen benzene at temperatures above 50 °C.[42][43]
Skin irritation
In a 1977, study using a human maximization test, 76% of subjects acquired a contact sensitization to benzoyl peroxide. Formulations of 5% and 10% were used.[44]
The US National Institute for Occupational Safety and Health has developed criteria for a recommended standard for occupational exposure to benzoyl peroxide.[45]
Cloth bleaching

Contact with fabrics or hair, such as from still-moist acne/pimple medication, can cause permanent color dampening almost immediately. Even secondary contact can cause bleaching, for example, contact with a towel that has been used to wash off benzoyl peroxide-containing hygiene products.[46]
References
- ↑ "Therapeutic Goods (Poisons Standard— June 2025) Instrument 2025" (pdf). May 2025. https://www.legislation.gov.au/F2025L00599/asmade/2025-05-28/text/original/pdf.
- ↑ "Epsolay- benzoyl peroxide cream". 25 April 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=507e2cd7-6bcf-4606-bc62-694d64537701.
- ↑ "Epsolay- benzoyl peroxide cream". 5 May 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2bb9bab5-770e-4e91-8d51-b9750454daf8.
- ↑ IUPAC Chemical Nomenclature and Structure Representation Division (2013). "P-65.7.5". in Favre, Henri A.; Powell, Warren H.. Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. IUPAC–RSC. ISBN 978-0-85404-182-4. https://pubs.rsc.org/en/Content/eBook/978-0-85404-182-4.
- ↑ 5.0 5.1 5.2 "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a19_199.
- ↑ 6.0 6.1 (in en) Encyclopaedia of Occupational Health and Safety: Guides, indexes, directory. International Labour Organization. 1998. p. 104. ISBN 978-92-2-109817-1. https://books.google.com/books?id=e4_S46UcI2AC&pg=PT404.
- ↑ 7.0 7.1 7.2 7.3 7.4 (in en) ACNE and ROSACEA (3 ed.). Springer Science & Business Media. 2012. p. 613. ISBN 978-3-642-59715-2. https://books.google.com/books?id=0cD-CAAAQBAJ&pg=PA613.
- ↑ 8.0 8.1 (in en) Alcamo's Fundamentals of Microbiology: Body Systems. Jones & Bartlett Publishers. 2012. p. 214. ISBN 978-1-4496-0595-7. https://books.google.com/books?id=cRaMAQAAQBAJ&pg=PA214.
- ↑ 9.0 9.1 WHO Model Formulary 2008. World Health Organization. 2009. pp. 307–308. ISBN 978-92-4-154765-9.
- ↑ 10.0 10.1 British national formulary : BNF 69 (69 ed.). British Medical Association. 2015. p. 820. ISBN 978-0-85711-156-2.
- ↑ (in en) Dermatology (2 ed.). Springer Science & Business Media. 2012. p. 1039. ISBN 978-3-642-97931-6. https://books.google.com/books?id=kK_rCAAAQBAJ&pg=PA1039.
- ↑ The selection and use of essential medicines 2023: web annex A: World Health Organization model list of essential medicines: 23rd list (2023). Geneva: World Health Organization. 2023. WHO/MHP/HPS/EML/2023.02.
- ↑ 13.0 13.1 Tarascon Pocket Pharmacopoeia 2015 Deluxe Lab-Coat Edition. Jones & Bartlett Learning. 2015. p. 173. ISBN 978-1-284-05756-0.
- ↑ "The Top 300 of 2021". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Benzoyl Peroxide - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/BenzoylPeroxide.
- ↑ 16.0 16.1 "Ueber die Bildung der Hyperoxyde organischer Säureradicale". Justus Liebigs Ann. Chem. 108: 79–83. 1858. doi:10.1002/jlac.18581080117. https://zenodo.org/record/1427113. Retrieved 2 July 2019.
- ↑ "Donald Holroyde Hey. 12 September 1904-21 January 1987". Biographical Memoirs of Fellows of the Royal Society 34: 294–320. 1988. doi:10.1098/rsbm.1988.0011.
- ↑ "432. Amphoteric aromatic substitution. Part II. Reactions of benzoyl peroxide and phenylazotriphenylmethane". Journal of the Chemical Society (Resumed): 1966. 1934. doi:10.1039/JR9340001966.
- ↑ The Welsh Academy Encyclopaedia of Wales. Cardiff: University of Wales Press. 2008. pp. 367–368. ISBN 978-0-7083-1953-6.
- ↑ "Radical pair in crystalline dibenzoyl peroxide evidence for triplet ground states". Tetrahedron 38 (6): 765–775. 1982. doi:10.1016/0040-4020(82)80157-9.
- ↑ Li III H (1998). "Chapter 2" (PDF). Synthesis, Characterization and Properties of Vinyl Ester Matrix Resins (Ph.D.). University of Vermont. hdl:10919/30521. Archived from the original on 20 September 2006. Retrieved 17 February 2007.
- ↑ "On the Nature of Certain Oxidizing Ferments". American Chemical Journal 2: 539–566. 1901.
- ↑ "Benzoylsuperoxyds, ein neues therapeutisches Agens" (in German). Therap Monatscheftel 12: 426–428. 1905.
- ↑ 24.0 24.1 "Benzoyl peroxide: a history of early research and researchers". International Journal of Dermatology 41 (3): 185–8. March 2002. doi:10.1046/j.1365-4362.2002.01371.x. PMID 12010349.
- ↑ 25.0 25.1 25.2 International Agency for Research on Cancer (1999): "Benzoyl peroxide". in Re-evaluation of Some Organic Chemicals, Hydrazine and Hydrogen Peroxide . Monographs on the Evaluation of Carcinogenic Risks to Humans, number 71, pages 345–358. ISBN 92-832-1271-1
- ↑ "Initiation By Diacyl Peroxides". https://polymerdatabase.com/polymer%20chemistry/Diaroyl%20Peroxides.html.
- ↑ "Error - Evonik Industries AG". http://www.degaroute.com/sites/dc/Downloadcenter/Evonik/Product/DEGAROUTE/en/Degaroute%20Brosch%C3%BCre.pdf.
- ↑ 28.0 28.1 28.2 28.3 "Newer approaches to the treatment of acne vulgaris". American Journal of Clinical Dermatology 13 (6): 357–64. December 2012. doi:10.2165/11632500-000000000-00000. PMID 22920095.
- ↑ 29.0 29.1 "Meta-analysis comparing efficacy of benzoyl peroxide, clindamycin, benzoyl peroxide with salicylic acid, and combination benzoyl peroxide/clindamycin in acne". Journal of the American Academy of Dermatology 63 (1): 52–62. July 2010. doi:10.1016/j.jaad.2009.07.052. PMID 20488582.
- ↑ "Adapalene". StatPearls. Treasure Island (FL): StatPearls Publishing. 2022. https://www.ncbi.nlm.nih.gov/books/NBK482509/. Retrieved 24 July 2022.
- ↑ 31.0 31.1 "Benzoyl peroxide". Acta Dermato-Venereologica. Supplementum 89: 57–63. 1 January 1980. doi:10.2340/00015555895763. PMID 6162349.
- ↑ 32.0 32.1 32.2 "[Acne therapy with topical benzoyl peroxide, antibiotics and azelaic acid]" (in German). Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology 4 (4): 293–300. April 2006. doi:10.1111/j.1610-0387.2006.05931.x. PMID 16638058.
- ↑ 33.0 33.1 Applied Therapeutics: The Clinical Use of Drugs (10th ed.). Baltimore: Wolters Kluwer Health/Lippincott Williams & Wilkins. 2013. p. 949. ISBN 978-1-60913-713-7.
- ↑ "Benzoyl peroxide: lack of sensitization". Acta Dermato-Venereologica 62 (5): 458–9. 1982. doi:10.2340/0001555562458459. PMID 6183909.
- ↑ "Benzoyl peroxide". Mayo Clinic. 1 January 2016. http://www.mayoclinic.org/drugs-supplements/benzoyl-peroxide-topical-route/side-effects/drg-20062425.
- ↑ "[Phototoxic activity of 5% benzoyl peroxide in man. Use of a new methodology]". Dermatologica 167 (1): 19–23. 1 January 1983. doi:10.1159/000249739. PMID 6628794.
- ↑ "Benzoyl Peroxide: Side Effects, Uses, Dosage, Interactions, Warnings" (in en). https://www.rxlist.com/benzoyl_peroxide/generic-drug.htm.
- ↑ "Some Facts On Teeth Whitening". https://worldofdentistry.co.in/some-facts-on-teeth-whitening.php.
- ↑ "Chemical Safety Data: Benzoyl peroxide". Oxford University. 17 March 2005. http://cartwright.chem.ox.ac.uk/hsci/chemicals/benzoyl_peroxide.html.
- ↑ Benzoyl peroxide, SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, April 2004, http://www.inchem.org/documents/sids/sids/BENZOYLPER.pdf
- ↑ "Skin tumor-promoting activity of benzoyl peroxide, a widely used free radical-generating compound". Science 213 (4511): 1023–5. August 1981. doi:10.1126/science.6791284. PMID 6791284. Bibcode: 1981Sci...213.1023S.
- ↑ "Benzoyl Peroxide Drug Products Form Benzene". Environmental Health Perspectives 132 (3): 37702. March 2024. doi:10.1289/EHP13984. PMID 38483533. Bibcode: 2024EnvHP.132c7702K.
- ↑ "USP Statement on Third Party Laboratory Benzene Findings | USP" (in en). https://www.usp.org/news/statement-on-third-party-laboratory-benzene-findings.
- ↑ "Contact sensitization to benzoyl peroxide". Contact Dermatitis 3 (5): 273–5. October 1977. doi:10.1111/j.1600-0536.1977.tb03674.x. PMID 145346.
- ↑ Criteria for a Recommended Standard: Occupational Exposure to Benzoyl Peroxide (77-166). 6 June 2014. doi:10.26616/NIOSHPUB76128. https://www.cdc.gov/niosh/docs/77-166/default.html. Retrieved 15 July 2016.
- ↑ "The short-term treatment of acne vulgaris with benzoyl peroxide: effects on the surface and follicular cutaneous microflora". The British Journal of Dermatology 132 (2): 204–8. February 1995. doi:10.1111/j.1365-2133.1995.tb05014.x. PMID 7888356.
External links
- International Chemical Safety Card 0225
- NIOSH Pocket Guide to Chemical Hazards. "#0052". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0052.html.
- SIDS Initial Assessment Report from the Organisation for Economic Co-operation and Development (OECD)
 |
