Chemistry:6-Methylisoxanthopterin
From HandWiki
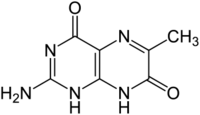
| |
| Names | |
|---|---|
| IUPAC name
2-Amino-6-methyl-1,8-dihydropteridine-4,7-dione
| |
| Other names
6-MI; 6MI
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H7N5O2 | |
| Molar mass | 193.166 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
6-Methylisoxanthopterin (6MI) is a base analog for the nucleotide guanine. It is useful as a fluorescent indicator because unlike most other base analogs, quenching does not occur when it is incorporated into a double helix. In fact, it exhibits a 3 to 4-fold increase in quantum yield when it is incorporated into a duplex formation.[1] This allows 6MI to be used to probe the dynamics of DNA or RNA helices using a technique such as fluorescence polarization anisotropy. [2]
See also
References
- ↑ Moreno, Andrew (2016). "Photophysical Characterization of Enhanced 6-Methylisoxanthopterin Fluorescence in Duplex DNA". The Journal of Physical Chemistry B 120 (48): 12232–12248. doi:10.1021/acs.jpcb.6b07369. PMID 27934220.
- ↑ Shi, Xuesong (2009). "Probing the Dynamics of the P1 Helix within the Tetrahymena Group I Intron". Journal of the American Chemical Society 131 (27): 9571–9578. doi:10.1021/ja902797j. PMID 19537712.
 |

