Chemistry:8-Hydroxygeraniol
From HandWiki
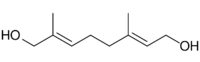
| |
| Names | |
|---|---|
| Preferred IUPAC name
(2E,6E)-2,6-Dimethylocta-2,6-diene-1,8-diol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C10H18O2 | |
| Molar mass | 170.252 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
8-Hydroxygeraniol (also incorrectly called 10-hydroxygeraniol)[1] is a monoterpene synthesized from geraniol by the enzyme geraniol 8-hydroxylase. 8-Hydroxygeraniol is a substrate for 8-hydroxygeraniol dehydrogenase (G80) which synthesizes 8-oxogeranial.[2] 8-Hydroxygeraniol is step in the synthesis of the secologanin, a key monoterpene needed for formation of terpene indole alkaloids.
In the laboratory, 8-hydroxygeraniol can be prepared from geranyl acetate.[3]
References
- ↑ Paul M. Dewick (2009). Medicinal Natural Products: A Biosynthetic Approach. doi:10.1002/9780470742761. ISBN 9780470742761.
- ↑ Miettinen, Karel; Dong, Lemeng; Navrot, Nicolas; Schneider, Thomas; Burlat, Vincent; Pollier, Jacob; Woittiez, Lotte; Van Der Krol, Sander et al. (2014). "The seco-iridoid pathway from Catharanthus roseus". Nature Communications 5: 3606. doi:10.1038/ncomms4606. PMID 24710322. Bibcode: 2014NatCo...5.....M.
- ↑ Ippoliti, Francesca M.; Barber, Joyann S.; Tang, Yi; Garg, Neil K. (2018). "Synthesis of 8-Hydroxygeraniol". The Journal of Organic Chemistry 83 (18): 11323–11326. doi:10.1021/acs.joc.8b01544. PMID 29969566.
 |
