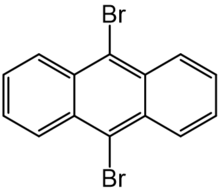
Chemistry:9,10-Dibromoanthracene
| |
| Names | |
|---|---|
| Preferred IUPAC name
9,10-Dibromoanthracene | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H8Br2 | |
| Molar mass | 336.026 g·mol−1 |
| Hazards | |
| GHS pictograms |  
|
| GHS Signal word | Warning |
| H315, H319, H335, H410 | |
| P261, P264, P271, P273, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P391, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
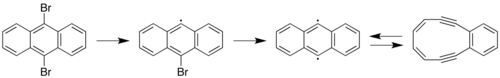
9,10-Dibromoanthracene is an organic chemical compound containing anthracene with two bromine atoms substituted on its central ring. It is notable in that it was the first single molecule to have a chemical reaction observed by an atomic force microscope and scanning tunneling microscopy.[1]
Production
Ian M. Heilbron and John S. Heaton were the first to synthesize this in 1923 in England.[1]
Properties
9,10-Dibromoanthracene is electroluminescent, giving off a blue light.[2]
Reactions
The carbon–bromine bonds can be fragmented in two successive steps by voltage pulses from tip of a scanning tunneling microscope. The resulting carbon radicals are stabilized by the sodium chloride substrate on which the 9,10-dibromoanthracene reactant was placed. Further voltage pulses cause the diradical to convert to a diyne (or back again) via a Bergman cyclization reaction.[3]
References
- ↑ 1.0 1.1 "9,10-Dibromoanthracene" (in en). https://www.acs.org/content/acs/en/molecule-of-the-week/archive/d/9-10-dibromoanthracene.html.
- ↑ Brar, Sukhwinder Singh; Mahajan, Aman; Bedi, R. K. (10 January 2014). "Structural, optical and electrical characterization of hot wall grown 9,10-dibromoanthracene films for light emitting applications". Electronic Materials Letters 10 (1): 199–204. doi:10.1007/s13391-013-3153-8.
- ↑ Borman, Stu (2016). "Chemists Nudge Molecule To React Then Watch Bonds Break And Form". 94. p. 7. https://cen.acs.org/articles/94/i5/Chemists-Nudge-Molecule-React-Watch.html.
 |