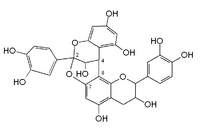
Chemistry:A-type proanthocyanidin
A type proanthocyanidins are a specific type of proanthocyanidins, which are a class of flavonoid. Proanthocyanidins fall under a wide range of names in the nutritional and scientific vernacular, including oligomeric proanthocyanidins, flavonoids, polyphenols, condensed tannins, and OPCs. Proanthocyanidins were first popularized by French scientist Jacques Masquelier.[1]
Distribution in plants
A-type linkage is a less common feature in proanthocyanidins with both 4β→8 (B-type) and 2β→O→7 interflavanoid bonds.[2]
A-type proanthocyanidin glycosides can be isolated from cocoa liquor.[3]
Dimers
- Procyanidin A1 is an epicatechin-(2β→7,4β→8)-catechin dimer.
- Procyanidin A2 is a dimeric (-)epicatechin.
Other A-type proanthocyanidins can be found in cranberries,[2] cinnamon,[4] peanut skins[5][6] and Geranium niveum.[7]
Chemistry
B-type procyanidins (catechin dimers) can be converted to A-type procyanidins by radical oxidation.[8] Fragmentation patterns for A-type proanthocyanidins include heterocyclic ring fission (HRF), retro-Diels-Alder (RDA) fission, benzofuran-forming fission (BFF) and quinone methide fission (QM).[9]
Metabolism
The metabolism of type-A proanthocyanidins is significant since a large number of metabolites are detected in urine and feces soon after ingestion of foods rich in polymers, indicating rapid elimination and absence of physiological effect. Polymeric type-A proanthocyanidins are depolymerized into epicatechin units in the small intestine, then cleaved into smaller phenolic acids with no known biological role.[10]
Research
In vitro, A-type proanthocyanidins isolated from cranberry juice cocktail demonstrated anti-adhesion activity against E. coli binding to urinary tract epithelial cells, whereas B-type proanthocyanidins from grape exhibited minor activity.[11] In humans, a 2014 review indicated there was insufficient clinical evidence that cranberry type-A proanthocyanidins are effective in lowering the risk of urinary tract infections (UTIs),[12] while a 2023 review concluded that long-term consumption of cranberry products may reduce the risk of UTIs in certain groups.[13]
References
- ↑ Fine, AM (2000). "Oligomeric proanthocyanidin complexes: history, structure, and phytopharmaceutical applications". Alternative Medicine Review 5 (2): 144–51. PMID 10767669. http://www.chiroonline.net/_fileCabinet/opc.pdf. Retrieved 22 September 2009.
- ↑ 2.0 2.1 Neto, CC (2007). "Cranberry and its phytochemicals: a review of in vitro anticancer studies". The Journal of Nutrition 137 (1 Suppl): 186S–193S. doi:10.1093/jn/137.1.186S. PMID 17182824.
- ↑ Hatano, T; Miyatake, H; Natsume, M; Osakabe, N; Takizawa, T; Ito, H; Yoshida, T (2002). "Proanthocyanidin glycosides and related polyphenols from cacao liquor and their antioxidant effects". Phytochemistry 59 (7): 749–58. doi:10.1016/S0031-9422(02)00051-1. PMID 11909632.
- ↑ María Luisa Mateos-Martín; Elisabet Fuguet; Carmen Quero; Jara Pérez-Jiménez; Josep Lluís Torres (2012). "New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI-TOF/TOF mass spectrometry". Analytical and Bioanalytical Chemistry 402 (3): 1327–1336. doi:10.1007/s00216-011-5557-3. PMID 22101466.
- ↑ de Camargo, A. C.; Regitano-d'Arce, M. A. B.; Gallo, C. R.; Shahidi, F. (2015). "Gamma-irradiation induced changes in microbiological status, phenolic profile and antioxidant activity of peanut skin". Journal of Functional Foods 12: 129–143. doi:10.1016/j.jff.2014.10.034.
- ↑ Hongxiang Lou; Yamazaku Y.; Sasaku T.; Uchida M.; Tanaka H.; Oka S. (1999). "A-type proanthocyanidins from peanut skins". Phytochemistry 51 (2): 297–308. doi:10.1016/S0031-9422(98)00736-5. http://cat.inist.fr/?aModele=afficheN&cpsidt=1831517.
- ↑ Calzada, F; Cerda-García-Rojas, CM; Meckes, M; Cedillo-Rivera, R; Bye, R; Mata, R (1999). "Geranins a and B, new antiprotozoal A-type proanthocyanidins from Geranium niveum". Journal of Natural Products 62 (5): 705–9. doi:10.1021/np980467b. PMID 10346950.
- ↑ Kondo, Kazunari; Kurihara, Masaaki; Fukuhara, Kiyoshi; Tanaka, Takashi; Suzuki, Takashi; Miyata, Naoki; Toyoda, Masatake (2000). "Conversion of procyanidin B-type (catechin dimer) to A-type: Evidence for abstraction of C-2 hydrogen in catechin during radical oxidation". Tetrahedron Letters 41 (4): 485–488. doi:10.1016/S0040-4039(99)02097-3.
- ↑ Li, Hui-Jing; Deinzer, Max L. (2008). "The mass spectral analysis of isolated hops A-type proanthocyanidins by electrospray ionization tandem mass spectrometry". Journal of Mass Spectrometry 43 (10): 1353–63. doi:10.1002/jms.1411. PMID 18416438.
- ↑ María Luisa Mateos-Martín; Jara Pérez-Jiménez; Elisabet Fuguet; Josep Lluís Torres (2012). "Profile of urinary and fecal proanthocyanidin metabolites from common cinnamon (Cinnamomum zeylanicum L.) in rats". Mol. Nutr. Food Res. 56 (4): 671–675. doi:10.1002/mnfr.201100672. PMID 22383303.
- ↑ "A-type cranberry proanthocyanidins and uropathogenic bacterial anti-adhesion activity". Phytochemistry 66 (18): 2281–91. 2005. doi:10.1016/j.phytochem.2005.05.022. PMID 16055161.
- ↑ "Scientific Opinion on the substantiation of a health claim related to CranMax® and reduction of the risk of urinary tract infection by inhibiting the adhesion of certain bacteria in the urinary tract pursuant to Article 14 of Regulation (EC) No 1924/20061". EFSA Journal 12 (5): 3657. 2014. doi:10.2903/j.efsa.2014.3657.
- ↑ Williams, Gabrielle; Hahn, Deirdre; Stephens, Jacqueline H.; Craig, Jonathan C.; Hodson, Elisabeth M. (2023-04-17). "Cranberries for preventing urinary tract infections". The Cochrane Database of Systematic Reviews 4 (4): CD001321. doi:10.1002/14651858.CD001321.pub6. ISSN 1469-493X. PMID 37068952.
 |