Chemistry:ABT-510
| |
| Names | |
|---|---|
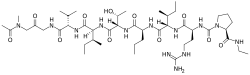
| IUPAC name
(2S)-1-[(2S)-2-[[(2S,3S)-2-[[(2S)-2-[[(2S,3R)-2-[[(2R,3S)-2-[[(2S)-2-[[2-[[2-[acetyl(methyl)amino]acetyl]amino]acetyl]amino]-3-methylbutanoyl]amino]-3-methylpentanoyl]amino]-3-hydroxybutanoyl]amino]pentanoyl]amino]-3-methylpentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]-N-ethylpyrrolidine-2-carboxamide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C46H83N13O11 | |
| Molar mass | 994.250 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
ABT-510 is a molecular therapeutic drug that was the subject of research as a potential treatment for cancer. According to the Journal of Clinical Oncology, ABT-510 is a "subcutaneously (SC) administered nonapeptide thrombospondin analogue."[1][2]
Following inconclusive phase I clinical trials, a 2007 phase II study of ABT-510 for treatment of metastatic melanoma failed to reach its primary endpoint resulting in termination of the study. Only three out of twenty-one patients reached the primary endpoint of progression-free survival at 18 weeks, but these three patients remained progression-free for 21, 34, and 42 weeks. However, biomarker data collected during this study showed a decrease in VEGF-C, circulating endothelial cells, and CD146 and CD34/133 counts, and a maximum tolerated dose has still not been established. Further study could consider a higher dose and/or combination treatment.[3]
References
- ↑ "2004 ASCO Annual Meeting Proceedings (Post-Meeting Edition)". J. Clin. Oncol. 22 (14S Suppl). July 15, 2004. 3080.
- ↑ NCI: ABT-510
- ↑ "A phase II study of ABT-510 (thrombospondin-1 analog) for the treatment of metastatic melanoma". Am. J. Clin. Oncol. 30 (3): 303–9. June 2007. doi:10.1097/01.coc.0000256104.80089.35. PMID 17551310.
 |

