Chemistry:ADA (buffer)
From HandWiki
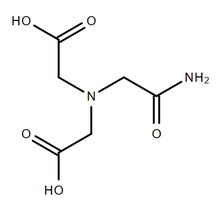
| |
| Names | |
|---|---|
| Systematic IUPAC name
2,2′-[(2-amino-2-oxoethyl)azanediyl]diacetic acid | |
| Other names
ADA, N-(2-acetamido)iminodiacetic acid, N-(carbamoylmethyl)iminodiacetic acid
| |
| Identifiers | |
3D model (JSmol)
|
|
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C6H10N2O5 | |
| Molar mass | 190.155 g/mol |
| Acidity (pKa) | 6.6 |
| Hazards | |
| Main hazards | Irritant |
| GHS Signal word | Warning |
| H302, H315, H319, H335 | |
| P261, P264, P270, P271, P280, P301+312, P302+352, P304+340, P305+351+338, P312, P321, P330, P332+313, P337+313, P362, P403+233, P405, P501 | |
| Flash point | Non-flammable |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
ADA is a zwitterionic organic chemical buffering agent; one of Good's buffers. It has a useful pH range of 6.0-7.2 in the physiological range, making it useful for cell culture work. It has a pKa of 6.6 with ΔpKa/°C of -0.011 and is most often prepared in 1 M NaOH where it has a solubility of 160 mg/mL.
ADA has been used in protein-free media for chicken embryo fibroblasts, as a chelating agent for H+, Ca2+, and Mg2+, and for isoelectric focusing in immobilized pH gradients. Its effects on dog kidney Na+/K+-ATPase and rat brain GABA receptors have also been studied. ADA does, however, alter coloring in bicinchoninic acid assays.[1]
References
 |
