Chemistry:AG 489
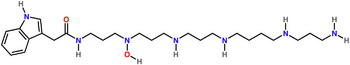
| |
| Names | |
|---|---|
| Preferred IUPAC name
N-(20-Amino-4-hydroxy-4,8,12,17-tetraazaicosan-1-yl)-2-(9H-purin-3-yl)acetamide | |
| Other names
Agatoxin 489
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C26H47N7O2 | |
| Molar mass | 489.69708 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
AG 489 (or agatoxin 489) is a component of the venom produced by Agelenopsis aperta,[1] a North American funnel web spider. It inhibits the ligand gated ion channel TRPV1 through a pore blocking mechanism.[2]
To identify new inhibitors, capsaicin receptor channels (TRPV1) were screened from a venom library for activity against these channels. In result, the robust inhibitory activity was found in the venom. Venom fractionation utilizing a reversed phase HPLC [2] which led to the purification of the two acylpolyamine toxins, AG489 and AG505. Both of these inhibit the TRPV1 channels [3] from the extracellular membrane side. From the pore blocking mechanism, the pore mutations that change toxic affinity were identified. As a result, the four mutants decreased toxic affinity and several mutants increased it. Therefore, this was consistent with the scanned TM5-TM6 linker region [4] being the outer vestibule of the channels and further confirming that AG489 is a pore blocker.
See also
References
- ↑ "Anesthesia and muscle relaxation with intrathecal injections of AR636 and AG489, two acylpolyamine spider toxins, in rat". Anesthesiology 77 (3): 507–12. September 1992. doi:10.1097/00000542-199209000-00016. PMID 1519789.
- ↑ 2.0 2.1 "An inhibitor of TRPV1 channels isolated from funnel Web spider venom". Biochemistry 44 (47): 15544–9. November 2005. doi:10.1021/bi051494l. PMID 16300403.
- ↑ "Transient receptor potential (TRP) channels: a clinical perspective". British Journal of Pharmacology 171 (10): 2474–507. May 2014. doi:10.1111/bph.12414. PMID 24102319.
- ↑ "Functionally important amino acid residues in the transient receptor potential vanilloid 1 (TRPV1) ion channel--an overview of the current mutational data". Molecular Pain 9: 30. June 2013. doi:10.1186/1744-8069-9-30. PMID 23800232.
External links
- AG+489 at the US National Library of Medicine Medical Subject Headings (MeSH)

