Chemistry:AP20187
From HandWiki
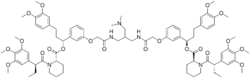
| |
| Names | |
|---|---|
| IUPAC name
2,2′-2-[(dimethylamino)methyl]-1,3-propanediyl]bis[imino(2-oxo-2,1-ethanediyl)oxy-3,1-phenylene[(1R)-3-(3,4-dimethoxyphenyl)propylidene] ester
| |
| Other names
(2S,2′S)-1-[(2S)-1-oxo-2-(3,4,5-trimethoxyphenyl)butyl]-2-piperidinecarboxylic acid
B/B Homodimerizer | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
PubChem CID
|
|
| |
| |
| Properties | |
| C82H107N5O20 | |
| Molar mass | 1482.773 g·mol−1 |
| Appearance | White to beige powder |
| Solubility | 2 mg/mL in DMSO[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
AP20187 is a ligand that can induce homodimerization of fusion proteins containing the F36V mutant of an FKBP domain(FKBPF36V),[2] available commercially as the DmrB domain.[3][4]
References
- ↑ MilliporeSigma (May 5, 2020). "SAFETY DATA SHEET AP20187". https://www.sigmaaldrich.com/US/en/sds/sigma/sml2838.
- ↑ Kawahara, Masahiro; Mabe, Satoru; Nagamune, Teruyuki (2014). "Detecting protein–protein interactions based on kinase-mediated growth induction of mammalian cells". Scientific Reports 4: 6127. doi:10.1038/srep06127. PMID 25135216.
- ↑ "Dimerizer Ligands - B/B Homodimerizer (AP20187), A/C Heterodimerizer (AP21967), D/D Solubilizer (AP21998), B/B Washout Ligand". http://www.clontech.com/US/Products/Inducible_Systems/Protein-Protein_Interactions/Dimerizer_Ligands. Retrieved February 5, 2016.
- ↑ Cotugno, G; Formisano, P; Giacco, F; Colella, P; Beguinot, F; Auricchio, A (2007). "AP20187-mediated activation of a chimeric insulin receptor results in insulin-like actions in skeletal muscle and liver of diabetic mice". Hum Gene Ther 18 (2): 106–17. doi:10.1089/hum.2006.116. PMID 17328681.
 |
