Chemistry:Abaucin
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
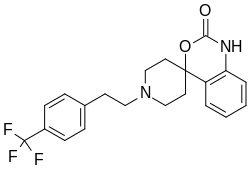
| Formula | C21H21F3N2O2 |
| Molar mass | 390.406 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Abaucin (RS-102895, MLJS-21001) is a compound which has been reported to show useful activity as a narrow-spectrum antibiotic.[1] There is evidence that it is effective against Acinetobacter baumannii, which is one of the three superbugs identified by the World Health Organization as a "critical threat" to humanity. Notably, abaucin was developed with assistance from artificial intelligence by a team led by the MIT Jameel Clinic's faculty lead for life sciences, James J. Collins, and McMaster's Jonathan Stokes.[2][3][4] Its mode of action involves inhibiting lipoprotein transport. The compound had previously been reported as an antagonist of the chemokine receptor CCR2, but its antibiotic activity was not discovered during earlier research.[5][6][7][8][9]
A The New York Times opinion piece by Peter Coy for Thanksgiving listed abaucin among scientific discoveries to be thankful for in 2023.[10]
See also
References
- ↑ "Deep learning-guided discovery of an antibiotic targeting Acinetobacter baumannii". Nature Chemical Biology 19 (11): 1342–1350. May 2023. doi:10.1038/s41589-023-01349-8. PMID 37231267.
- ↑ "New superbug-killing antibiotic discovered using AI". BBC News. May 25, 2023. https://www.bbc.com/news/health-65709834.
- ↑ "Using AI, scientists find a drug that could combat drug-resistant infections". MIT News. 25 May 2023. https://news.mit.edu/2023/using-ai-scientists-combat-drug-resistant-infections-0525.
- ↑ "Scientists use AI to find promising new antibiotic to fight evasive hospital superbug". McMaster University. 25 May 2023. https://brighterworld.mcmaster.ca/articles/artificial-intelligence-new-antibiotic-drug-resistant-pathogen-acinetobacter-baumannii/.
- ↑ "Synthesis and antihypertensive activity of 4'-substituted spiro[4H-3,1-benzoxazine-4,4'-piperidin]-2(1H)-ones". Journal of Medicinal Chemistry 26 (5): 657–661. May 1983. doi:10.1021/jm00359a007. PMID 6842505.
- ↑ "Identification of the binding site for a novel class of CCR2b chemokine receptor antagonists: binding to a common chemokine receptor motif within the helical bundle". The Journal of Biological Chemistry 275 (33): 25562–25571. August 2000. doi:10.1074/jbc.M000692200. PMID 10770925.
- ↑ "Ammonium pyrrolidine dithiocarbamate and RS 102895 attenuate opioid withdrawal in vivo and in vitro". Psychopharmacology 220 (2): 427–438. March 2012. doi:10.1007/s00213-011-2489-8. PMID 21931991.
- ↑ "CCR2 gene deletion and pharmacologic blockade ameliorate a severe murine experimental autoimmune neuritis model of Guillain-Barré syndrome". PLOS ONE 9 (3): e90463. 2014. doi:10.1371/journal.pone.0090463. PMID 24632828. Bibcode: 2014PLoSO...990463Y.
- ↑ "CCL2 mediates early renal leukocyte infiltration during salt-sensitive hypertension". American Journal of Physiology. Renal Physiology 318 (4): F982–F993. April 2020. doi:10.1152/ajprenal.00521.2019. PMID 32150444.
- ↑ Coy, Peter (2023-11-24). "Opinion | What Am I Thankful for This Year? Amazing Scientific Discoveries." (in en-US). The New York Times. ISSN 0362-4331. https://www.nytimes.com/2023/11/24/opinion/thanksgiving-science-technology.html.
 |
