Chemistry:Actinobolin
From HandWiki
| |
| Names | |
|---|---|
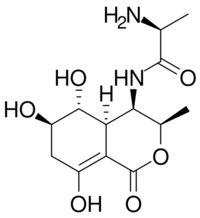
| IUPAC name
(2S)-2-Amino-N-[(3R,4R,4aR,5R,6R)-5,6,8-trihydroxy-3-methyl-1-oxo-3,4,4a,5,6,7-hexahydroisochromen-4-yl]propanamide[1]
| |
| Other names
(Propanamide, 2-amino-N-(3,4,4a,5,6,7-hexahydro-5,6, 8-trihydroxy-3-met hyl-1-oxo-1H-2-benzopyran-4-yl)-
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C13H20N2O2 | |
| Molar mass | 236.315 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Actinobolin is a antibiotic with the molecular formula C13H20N2O6.[1] Actinobolin is produced by the bacterium Streptomyces griseoviridus var atrofaciens.[2]
References
- ↑ 1.0 1.1 "Actinobolin" (in en). Pubchem.ncbi.NLM.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/Actinobolin#section=3D-Conformer.
- ↑ (in en) Studies in Natural Products Chemistry: Stereoselective Synthesis (Part J). Elsevier. 10 August 1995. p. 3. ISBN 978-0-08-054178-5.
Further reading
- Tharra, Prabhakara R.; Mikhaylov, Andrey A.; Švejkar, Jiří; Gysin, Marina; Hobbie, Sven N.; Švenda, Jakub (28 February 2022). "Short Synthesis of (+)-Actinobolin: Simple Entry to Complex Small-Molecule Inhibitors of Protein Synthesis". Angewandte Chemie International Edition 61 (17): e202116520. doi:10.1002/anie.202116520. PMID 35167723.
- (in en) Antimicrobial Agents and Chemotherapy. 1958. p. 503.
- Munk, Morton E.; Sodano, Charles S.; McLean, Robert L.; Haskell, Theodore H. (August 1967). "Actinobolin. I. Structure of actinobolamine". Journal of the American Chemical Society 89 (16): 4158–4165. doi:10.1021/ja00992a034. PMID 6045607.
 |

