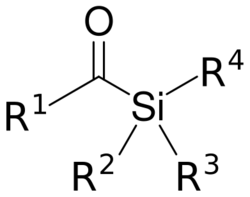
Chemistry:Acylsilane
Acylsilanes are a group of chemical compounds sharing a common functional group with the general structure R(CO)-SiR3.[1] Acylsilanes are starting compounds in the Brook rearrangement with vinyl lithium compounds to silyl enol ethers.
Synthesis
Acylsilanes can be synthesized by treating acyl anion equivalents with silyl halides (typically trimethylsilyl chloride, tmsCl). One route starts with silylation of the 2-lithio-1,3-dithiane, followed by removal of the dithioacetal group with mercury(II) chloride and hydrolysis.[2] This method also can make acylgermanes using the appropriate halogermane reagents.
Several approches to acylsilanes start with carboxylic acid derivatives.[1] Esters undergo reductive silylation en route to acylsilanes:
- RCO
2Me + 2 tmsCl + Mg → RC(OMe)(Otms)(tms) + MgCl
2 - RC(OMe)(Otms(tms) + → RC(O)tms + MeOtms
Tertiary amides react with silyl lithium reagents. Acid chlorides are converted using hexamethyldisilane:
- RCOCl + tms–tms → RCOtms + tmsCl
Some acyl silanes are prepared by oxidation of a suitable silanes.[1]

Further reading
- The reactivity of α- and β-iodo propenoylsilanes: an alternative access to polyunsaturated acylsilanes Alessandro Degl’Innocenti, Antonella Capperucci, Patrizia Scafato, Antonella Telesca Arkivoc 0-005A 2000 Article
References
- ↑ 1.0 1.1 1.2 Zhang, Hui-Jun; Priebbenow, Daniel L.; Bolm, Carsten (2013). "Acylsilanes: Valuable organosilicon reagents in organic synthesis". Chemical Society Reviews 42 (21): 8540–8571. doi:10.1039/c3cs60185d. PMID 23942548.
- ↑ Brook, A. G. (Jan 1, 1967). "Synthesis of Silyl and Germyl Ketones". Journal of the American Chemical Society 89 (2): 431–434. doi:10.1021/ja00978a047.
- ↑ *Kuwajima, Isao; Abe, Toru; Minami, Naoki (1976-09-05). "An Efficient Method for the Preparation of Acylsilane and α-haloacylsilane". Chemistry Letters 5 (9): 993–994. doi:10.1246/cl.1976.993. ISSN 0366-7022.
 |