Chemistry:Agosterol A
From HandWiki
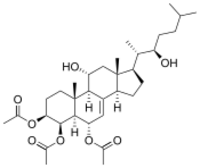
| |
| Names | |
|---|---|
| IUPAC name
(22R)-11α,22-Dihydroxy-5α-cholest-7-ene-3β,4β,6α-triyl triacetate
| |
| Systematic IUPAC name
(1R,3aR,5S,5aS,6R,7S,9aR,9bR,10R,11aR)-10-Hydroxy-1-[(2S,3R)-3-hydroxy-6-methylheptan-2-yl]-9a,11a-dimethyl-2,3,3a,5,5a,6,7,8,9,9a,9b,10,11,11a-tetradecahydro-1H-cyclopenta[a]phenanthrene-5,6,7-triyl triacetate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C33H52O8 | |
| Molar mass | 576.771 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Agosterol A is a bio-active sterol which may have applications in removing multi-drug resistance in various cancers.[1][2] It was first isolated from marine sponge but has also been produced synthetically.[3]
References
- ↑ Chen, Zhe-Sheng; Aoki, Shunji; Komatsu, Masaharu; Ueda, Kazumitsu; Sumizawa, Tomoyuki; Furukawa, Tatsuhiko; Okumura, Hiroshi; Ren, Xiao-Qin et al. (2001). "Reversal of drug resistance mediated by multidrug resistance protein (MRP) 1 by dual effects of agosterol a on MRP1 function". International Journal of Cancer 93 (1): 107–113. doi:10.1002/ijc.1290. ISSN 0020-7136. PMID 11391629.
- ↑ Aoki, Shunji; Chen, Zhe-Sheng; Higasiyama, Kimihiko; Setiawan, I; Akiyama, Shin-ichi; Kobayashi, Motomasa (2001). "Reversing Effect of Agosterol A, a Spongean Sterol Acetate, on Multidrug Resistance in Human Carcinoma Cells". Japanese Journal of Cancer Research 92 (8): 886–895. doi:10.1111/j.1349-7006.2001.tb01177.x. ISSN 0910-5050. PMID 11509122.
- ↑ Murakami, Nobutoshi; Sugimoto, Masanori; Morita, Mari; Kobayashi, Motomasa (2001). "Total Synthesis of Agosterol A: an MDR-Modulator from a Marine Sponge". Chemistry: A European Journal 7 (12): 2663–2670. doi:10.1002/1521-3765(20010618)7:12<2663::AID-CHEM26630>3.0.CO;2-U. ISSN 0947-6539. PMID 11465457.
 |
