Chemistry:Aldol–Tishchenko reaction
From HandWiki
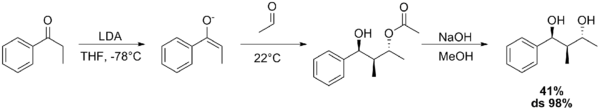
The Aldol–Tishchenko reaction is a tandem reaction involving an aldol reaction and a Tishchenko reaction. In organic synthesis it is a method to convert aldehydes and ketones into 1,3-hydroxyl compounds. The reaction sequence in many examples starts from conversion of a ketone into an enolate by action of lithium diisopropylamide (LDA), to which a suitable aldehyde is added. The resulting mono-ester diol is then converted into the diol by a hydrolysis step. With both the acetyl trimethylsilane[1] and propiophenone[2] as reactants, the diol is obtained as a pure diastereoisomer.
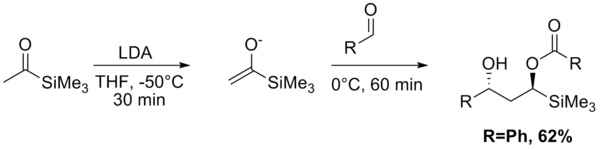
References
- ↑ Mitsunori Honda; Ryota Iwamoto; Yoshie Nogami; Masahito Segi (2005). "Stereoselective Tandem Aldol–Tishchenko Reaction with Acylsilanes". Chemistry Letters 34 (4): 466–467. doi:10.1246/cl.2005.466.
- ↑ Paul M. Bodnar; Jared T. Shaw; K. A. Woerpel (1997). "Tandem Aldol–Tishchenko Reactions of Lithium Enolates: A Highly Stereoselective Method for Diol and Triol Synthesis". Journal of Organic Chemistry 62 (17): 5674–5675. doi:10.1021/jo971012e. Supporting information
 |

