Chemistry:Alisertib
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
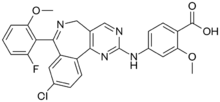
4-{[9-Chloro-7-(2-fluoro-6-methoxyphenyl)-5H-pyrimido[5,4-d][2]benzazepin-2-yl]amino}-2-methoxybenzoic acid | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C27H20ClFN4O4 | |
| Molar mass | 518.93 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Alisertib (MLN8237) is an orally available selective aurora A kinase inhibitor developed by Takeda.[1] It was investigated as a treatment for relapsed or refractory peripheral T-cell lymphoma.[2][3] Development was abandoned in 2015 due to poor clinical trial results.[4]
References
- ↑ Friedberg, JW; Mahadevan, D; Cebula, E; Persky, D; Lossos, I; Agarwal, AB; Jung, J; Burack, R et al. (Jan 1, 2014). "Phase II study of alisertib, a selective Aurora A kinase inhibitor, in relapsed and refractory aggressive B- and T-cell non-Hodgkin lymphomas.". Journal of Clinical Oncology 32 (1): 44–50. doi:10.1200/JCO.2012.46.8793. PMID 24043741.
- ↑ "Millennium Initiates Pivotal Phase 3 Trial of MLN8237 in Patients With Relapsed or Refractory Peripheral T-cell Lymphoma". Takeda Pharmaceutical Company Limited; Millennium Pharmaceuticals, Inc.. March 6, 2012. http://www.takeda.com/news/2012/20120306_3946.html. Retrieved 20 March 2014.
- ↑ "Research and Development Pipeline (As of February 5, 2014)". Takeda Pharmaceutical Company Limited. February 5, 2014. p. 2. http://www.takeda.com/research/files/pipeline_20140205_en.pdf. Retrieved 20 March 2014.
- ↑ "Takeda Announces Termination of Alisertib Phase 3 Trial in Relapsed or Refractory Peripheral T-cell Lymphoma". https://www.takeda.com/newsroom/newsreleases/2015/takeda-announces-termination-of-alisertib-phase-3-trial-in-relapsed-or-refractory-peripheral-t-cell-lymphoma/.
 |
