Chemistry:Alkylidene ketene
Alkylidene ketenes are a class of organic compounds that are of the form R2C=C=C=O. They are a member of the family of heterocumulenes (R2C=(C)n=O), and are often considered an unsaturated homolog of ketenes (R2C=C=O). Sometimes referred to as methyleneketenes, these compounds are highly reactive and much more difficult to access than ketenes. Because of their instability, alkylidene ketenes are often observed as reaction intermediates. While the parent alkylidene ketene propadienone only exists on the order of seconds in vacuum at room temperature,[1] other more highly substituted species are stable at room temperature.[2] A notable alkylidene ketene is carbon suboxide, of the structure O=C=C=C=O.[3]
Properties

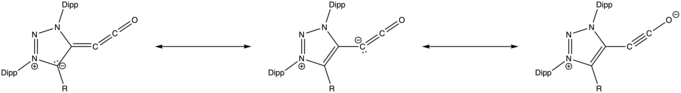
Despite the nature of the multiple double bonds between heavy atoms in alkylidene ketenes, they have been to shown to adopt a slightly bent geometry that is not truly fully linear. Brown et al. found via the microwave spectra of 13C-labelled propadienone the structural parameters for this species, along with observed intersystem transitions that show that it converts between equivalent bent conformations, resulting in a molecular nonrigidity.[4] More recent studies of 1,2,3-triazole and imidazole-based alkylidene ketenes have confirmed similar bent structures via X-ray crystallography.[2][5]
IR and NMR analysis of the room-temperature stable 1,2,3-triazole stabilized alkylidene ketene suggests three major resonance structures as shown below.[2]
The alkylidene ketene group gives strong IR peaks around 2100 and 2085 cm−1, similar to previously studied trapped methyleneketenes,[6] and indicating π-backbonding character into CO. 13C-NMR indicates a negative charge on the α-carbon, supporting the zwitterionic resonance structure (pictured center above).[2]
Synthesis
The most common synthesis for substituted alkylidene ketenes is via the thermolysis of an alkylidene derivative of Meldrum's acid.[7] Some other common synthetic routes are summarized below. Notably, in 2021, Severin and Hansmann both reported novel synthetic methods for room-temperature stable alkylidene ketenes via N2/CO exchange from diazoalkenes stabilized by N-heterocyclic carbenes.[2][5]
Thermolysis of carboxylic acids and Meldrum's acid species
These reactions are typically done in the gas phase. Elimination of an α,β-unsaturated carboxylic acid is difficult since it requires breaking the C-H bond of an sp2 hybridized carbon. Presently, using a Meldrum's acid derivative as a starting material is the most common synthetic route for synthesizing alkylidene ketenes.

Photochemical cleavage
Alkylidene ketenes can be generated by cleaving an α,β-unsaturated carbonyl or cyclic ketene with a combination of heat and irradiation. While this transformation can occur through thermolysis, this process proceeds much more easily via photoirradiation.[9]

center|449x449px|thumb|Ring opening of azetidinone to form methylene ketene[11]

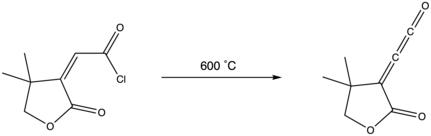
Via acid anhydride pyrolysis
Pyrolysis of anhydrides and intramolecular hydrogen transfer in a propiolic acid can also make alkylidene ketenes. This particular transformation is believed to go through a propiolaldehyde intermediate that generates acetylene via carbon monoxide loss.[13]

Via N2/CO exchange
Reacting N-heterocyclic olefins with N2O to afford various diazoolefin species, Severin and Hansmann reported a method for generating highly thermally stable alkylidene ketenes via N2/CO exchange at atmospheric pressure.[2][5]


Reactions
Dimerization
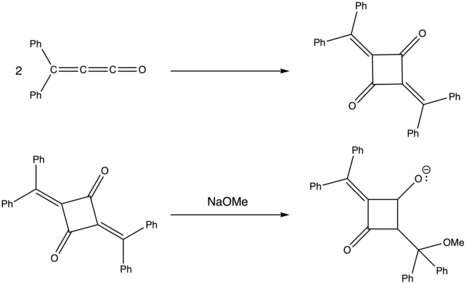
Alkylidene ketenes can readily dimerize and participate in cycloaddition reactions. Often orange or red in color, these dimers can be generated both in solution and via pyrolysis.[14] Dimer formation is typically inhibited at the low temperatures used to analyze monomer species (especially methylene ketene monomers), but once the dimers are formed, it is often impossible to convert back to its substituent monomers even via thermolysis at high temperatures.[15]
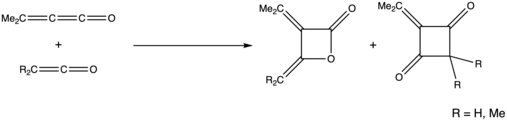
Cycloaddition
Various cycloadducts of alkylidene ketenes can be made, including the addition of an alkylidene ketene and ketene depicted below. When reacting with either ketene or dimethylketene, the formation of the β-lactone product was favored, as this cyclization occurs via an attack on the terminal ketene carbonyl.[16]
Reactions with nucleophiles
Alkylidene ketenes react similarly to ketenes in the presence of nucleophiles, often generating equal amounts of E and Z isomers in α,β-unsaturated esters. Secondary isomerism in pyrolytic systems can, however, result in the isolation of a thermodynamic product, as is the case with the generation of phenyl-substituted methylene ketene from a Meldrum's acid derivative and hot methanol vapor.[14] Other alkyl substitutions can also lead to β,γ-unsaturated products.[17] This migration of the double bond can occur via secondary photoenolization, deconjugation of unsaturated products, or isomerization to a vinylidene ketene.[18]
Decarbonylation
Decarbonylation has been observed but is thermodynamically difficult to achieve with an activation energy of over 40 kcal mol-1. However, the overall decarbonylation of propadienone to ethyne and carbon monoxide is exothermic by 2 - 5 kcal mol−1.[19]
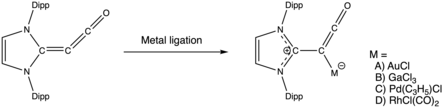
As C-donor ligand for transition and main group metals
Severin reported coordination chemistry using an imidazole-stabilized alkylidene ketene. Coordination increased the C-C-CO bond angle and bond lengths, indicating increased ylidic character.[20] Using the average CO stretching frequency as a measure of donor strength, the alkylidene ketene is weaker than its diazoolefin starting material, but stronger than N-heterocyclic carbenes.[21]
See also
References
- ↑ Brown, Roger F. C.; Eastwood, Frank W.; McMullen, Gabrielle L. (1976-10-01). "Evidence for the pyrolytic generation of methyleneketene (propadienone)" (in en). Journal of the American Chemical Society 98 (23): 7421–7422. doi:10.1021/ja00439a052. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00439a052.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Antoni, Patrick W.; Reitz, Justus; Hansmann, Max M. (2021-08-18). "N 2 /CO Exchange at a Vinylidene Carbon Center: Stable Alkylidene Ketenes and Alkylidene Thioketenes from 1,2,3-Triazole Derived Diazoalkenes" (in en). Journal of the American Chemical Society 143 (32): 12878–12885. doi:10.1021/jacs.1c06906. ISSN 0002-7863. PMID 34348463. https://pubs.acs.org/doi/10.1021/jacs.1c06906.
- ↑ "II. Note on the synthesis of marsh-gas and formic acid, and on the electric decomposition of carbonic oxide" (in en). Proceedings of the Royal Society of London 21 (139–147): 245–247. 1873-12-31. doi:10.1098/rspl.1872.0052. ISSN 0370-1662. https://royalsocietypublishing.org/doi/10.1098/rspl.1872.0052.
- ↑ 4.0 4.1 Brown, Ronald D.; Champion, Robert; Elmes, Patricia S.; Godfrey, Peter D. (1985-07-01). "The structure of propadienone" (in en). Journal of the American Chemical Society 107 (14): 4109–4112. doi:10.1021/ja00300a001. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00300a001.
- ↑ 5.0 5.1 5.2 5.3 5.4 Feuerstein, Wolfram; Varava, Paul; Fadaei-Tirani, Farzaneh; Scopelliti, Rosario; Severin, Kay (2021). "Synthesis, structural characterization, and coordination chemistry of imidazole-based alkylidene ketenes" (in en). Chemical Communications 57 (87): 11509–11512. doi:10.1039/D1CC05161J. ISSN 1359-7345. PMID 34652353. http://xlink.rsc.org/?DOI=D1CC05161J.
- ↑ 6.0 6.1 Lorenčak, Primoz; Pommelet, J. C.; Chuche, Josselin; Wentrup, Curt (1986-01-01). "A stable methyleneketene and the stepwise fragmentation of Meldrum's acids" (in en). Journal of the Chemical Society, Chemical Communications (5): 369–370. doi:10.1039/C39860000369. ISSN 0022-4936. https://pubs.rsc.org/en/content/articlelanding/1986/c3/c39860000369.
- ↑ Wentrup, Curt; Gross, Gerhard; Berstermann, Hans Michael; Lorencak, Primoz (1985-08-01). "Mechanism of fragmentation of alkylidene-Meldrum's acids. Carboxyketene, vinylketene, and methyleneketene intermediates from 5-cyclopentylidene-2,2-dimethyl-1,3-dioxane-4,6-dione" (in en). The Journal of Organic Chemistry 50 (16): 2877–2881. doi:10.1021/jo00216a015. ISSN 0022-3263. https://pubs.acs.org/doi/abs/10.1021/jo00216a015.
- ↑ Brown, R. F. C.; Coulston, K. J.; Eastwood, F. W.; Irvine, M. J. (1991). "Synthesis of Dimethylbutatrienone, (CH3)2C=C=C=C=O, by a Rearrangement-Fragmentation Process and by Direct Elimination" (in en). Australian Journal of Chemistry 44 (1): 87–101. doi:10.1071/ch9910087. ISSN 1445-0038. https://www.publish.csiro.au/ch/ch9910087.
- ↑ Pac, Chyongjin; Nakasone, Akira; Sakurai, Hiroshi (1977-08-01). "Photochemical reactions of aromatic compounds. 28. Photosensitized electron-transfer reaction of electron donor-acceptor pairs by aromatic hydrocarbons" (in en). Journal of the American Chemical Society 99 (17): 5806–5808. doi:10.1021/ja00459a049. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00459a049.
- ↑ Hart, Harold; Dean, David; Buchanan, Douglas (February 15, 1973). "Methyleneketenes by Irradiation of alpha,beta-Unsaturated Ketones". Journal of the American Chemical Society 95 (19): 6294–6301. doi:10.1021/ja00800a023. https://pubs.acs.org/doi/pdf/10.1021/ja00800a023.
- ↑ Mazzocchi, Paul; Bowen, Michael; Kachinsky, Joseph (1976-09-01). "Generation of an Aliphatic Methyleneketen. Photolysis of N-Methyl-4,4-dimethyl-3-isopropylideneazetidin-2-one". Journal of the Chemical Society, Chemical Communications (2): 53–54. doi:10.1039/C39770000053. https://pubs.rsc.org/en/content/articlelanding/1977/c3/c39770000053.
- ↑ Chapman, O. L.; Chang, C. C.; Kolc, J.; Rosenquist, N. R.; Tomioka, H. (1975-10-01). "Photochemical method for the introduction of strained multiple bonds. Benzyne C .tbd. C stretch" (in en). Journal of the American Chemical Society 97 (22): 6586–6588. doi:10.1021/ja00855a055. ISSN 0002-7863. https://pubs.acs.org/doi/abs/10.1021/ja00855a055.
- ↑ 13.0 13.1 Blackman, GL; Brown, RD; Brown, RFC; Eastwood, FW; McMullen, GL; Robertson, ML (1978). "Methyleneketenes and methylenecarbenes. X. Precursors for the generation of methyleneketene and deuterated methyleneketenes for microwave spectroscopy". Australian Journal of Chemistry 31 (1): 209. doi:10.1071/ch9780209. ISSN 0004-9425. http://dx.doi.org/10.1071/ch9780209.
- ↑ 14.0 14.1 Brown, RFC; Eastwood, FW; Harrington, KJ (1974). "Methyleneketenes and methylenecarbenes. I. Formation of arylmethyleneketenes and alkylideneketenes by pyrolysis of substituted 2,2-Dimethyl-1,3-dioxan-4,6-diones". Australian Journal of Chemistry 27 (11): 2373. doi:10.1071/ch9742373. ISSN 0004-9425. http://dx.doi.org/10.1071/ch9742373.
- ↑ 15.0 15.1 Taylor, G. A. (1969-01-01). "Keten. Part VII. A dimer of diphenylpropadienone" (in en). Journal of the Chemical Society C: Organic (13): 1755–1758. doi:10.1039/J39690001755. ISSN 0022-4952. https://pubs.rsc.org/en/content/articlelanding/1969/j3/j39690001755.
- ↑ 16.0 16.1 Baxter, GJ; Brown, RFC; Eastwood, FW; Gatehouse, BM; Nesbit, MC (1978). "Methyleneketenes and methylenecarbenes. XIII. Cycloaddition of ketenes and of a 1-pyrroline 1-oxide to isopropylideneketene". Australian Journal of Chemistry 31 (8): 1757. doi:10.1071/ch9781757. ISSN 0004-9425. http://dx.doi.org/10.1071/ch9781757.
- ↑ Patai, Saul, ed (1991). The Chemistry of ketenes, allenes and related compounds. 2. The chemistry of functional groups (Faks.-Druck 1991 ed.). Chichester: Wiley. ISBN 978-0-471-27670-8.
- ↑ Mazzocchi, Paul H.; Bowen, Michael W.; Kachinsky, Joseph (1977-01-01). "Generation of an aliphatic methyleneketen. Photolysis of N-methyl-4,4-dimethyl-3-isopropylideneazetidin-2-one" (in en). Journal of the Chemical Society, Chemical Communications (2): 53–54. doi:10.1039/C39770000053. ISSN 0022-4936. https://pubs.rsc.org/en/content/articlelanding/1977/c3/c39770000053.
- ↑ Radom, L (1978). "An ab initio molecular orbital study of the structure and properties of propadienone (methyleneketene)". Australian Journal of Chemistry 31 (1): 1. doi:10.1071/ch9780001. ISSN 0004-9425. http://dx.doi.org/10.1071/ch9780001.
- ↑ Munz, Dominik; Meyer, Karsten (2021-05-27). "Charge frustration in ligand design and functional group transfer". Nature Reviews Chemistry 5 (6): 422–439. doi:10.1038/s41570-021-00276-3. ISSN 2397-3358. http://dx.doi.org/10.1038/s41570-021-00276-3.
- ↑ Dröge, Thomas; Glorius, Frank (2010-09-17). "The Measure of All Rings—N‐Heterocyclic Carbenes" (in en). Angewandte Chemie International Edition 49 (39): 6940–6952. doi:10.1002/anie.201001865. ISSN 1433-7851. PMID 20715233. https://onlinelibrary.wiley.com/doi/10.1002/anie.201001865.
 |