Chemistry:Aluminium phenolate
From HandWiki
| |
| Names | |
|---|---|
| Other names
Aluminium phenoxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| |
| |
| Properties | |
| C18H15AlO3 | |
| Molar mass | 306.297 g·mol−1 |
| Appearance | white solid |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Danger |
| H314 | |
| P260, P264, P280, P301+330+331, P303+361+353, P304+340, P305+351+338, P310, P321, P363, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
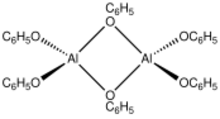
Aluminium phenolate is the metalloorganic compound with the formula [Al(OC6H5)3]n. It is a white solid. 27Al NMR studies suggest that aluminium phenolate exists in benzene solution as a mixture of dimer and trimer.[2] The compound can be prepared by the reaction of elemental aluminium with phenol:[3]
- Al + 3 HOC6H5 → Al(OC6H5)3 + 1.5 H2
The compound is used as a catalyst for the alkylation of phenols with various alkenes. For example, the ethylphenols are generated commercially by treating phenol with ethylene in the presence of a catalytic amount of aluminium phenolate.[4]
Related compounds
References
- ↑ "Aluminium triphenolate" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/167236#section=Safety-and-Hazards.
- ↑ Kříž, O.; Čásenský, B.; Lyčka, A.; Fusek, J.; Heřmánek, S. (1984). "27Al NMR Behavior of Aluminum Alkoxides". Journal of Magnetic Resonance 60 (3): 375–381. doi:10.1016/0022-2364(84)90048-9. Bibcode: 1984JMagR..60..375K.
- ↑ Kolka, Alfred J.; Napolitano, John P.; Filbey, Allen H.; Ecke, George G. (1957). "The ortho-Alkylation of Phenols". The Journal of Organic Chemistry 22 (6): 644. doi:10.1021/jo01357a014. "The aluminum phenoxide catalyst was prepared by adding 4.5 g. (1⁄6 formula wt.) of aluminum turnings in small amounts and with vigorous stirring to 300 g. of phenol at 165° under a nitrogen atmosphere.".
- ↑ Fiege, Helmut; Voges, Heinz-Werner; Hamamoto, Toshikazu; Umemura, Sumio; Iwata, Tadao; Miki, Hisaya; Fujita, Yasuhiro; Buysch, Hans-Josef et al. (2000). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. p. 533. doi:10.1002/14356007.a19_313.
 |
