Chemistry:Aminomethanol
From HandWiki
| |
| Names | |
|---|---|
| Preferred IUPAC name
Aminomethanol | |
| Other names
Methanolamine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| CH5NO | |
| Molar mass | 47.057 g·mol−1 |
| Appearance | Colorless liquid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
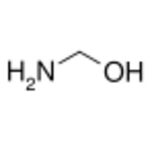
Aminomethanol or methanolamine is the amino alcohol with the chemical formula of H2NCH2OH. With an amino group and an alcohol group on the same carbon atom, the compound is also an hemiaminal.[1]
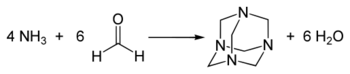
In aqueous solution, methanolamine exists in equilibrium with formaldehyde and ammonia.[2] It is an intermediate en route to hexamethylenetetramine.[3] The reaction can be conducted in gas phase and in solution.
References
- ↑ Berski, Sławomir; Gordon, Agnieszka J.; Ćmikiewicz, Agnieszka (2018-02-01). "Characterisation of the reaction mechanism between ammonia and formaldehyde from the topological analysis of ELF and catastrophe theory perspective" (in en). Structural Chemistry 29 (1): 243–255. doi:10.1007/s11224-017-1024-x. ISSN 1572-9001.
- ↑ T Feldmann, Michael; Widicus Weaver, Susanna; Blake, Geoffrey; R Kent, David; Goddard, William (2005-08-01). "Aminomethanol water elimination: Theoretical examination". The Journal of Chemical Physics 123 (3): 34304. doi:10.1063/1.1935510. PMID 16080734. Bibcode: 2005JChPh.123c4304F. https://www.researchgate.net/publication/7678861.
- ↑ Eller, K.; Henkes, E.; Rossbacher, R.; Höke, H. (2000). Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH. doi:10.1002/14356007.a02_001. ISBN 9783527306732.
 |