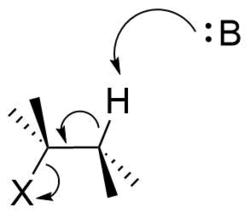Chemistry:Anti-periplanar
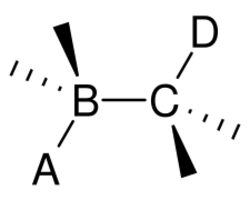
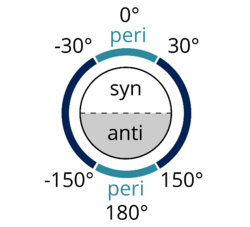
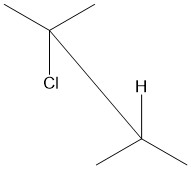
In organic chemistry, anti-periplanar, or antiperiplanar, describes the A–B–C–D bond angle in a molecule. In this conformer, the dihedral angle of the A–B bond and the C–D bond is greater than +150° or less than −150°[1] (Figures 1 and 2). Anti-periplanar is often used in textbooks to mean strictly anti-coplanar,[2] with an A–B C–D dihedral angle of 180° (Figure 3). In a Newman projection, the molecule will be in a staggered arrangement with the anti-periplanar functional groups pointing up and down, 180° away from each other (see Figure 4). Figure 5 shows 2-chloro-2,3-dimethylbutane in a sawhorse projection with chlorine and a hydrogen anti-periplanar to each other.
Syn-periplanar or synperiplanar is similar to anti-periplanar. In the syn-periplanar conformer, the A and D are on the same side of the plane of the bond, with the dihedral angle of A–B and C–D between +30° and −30° (see Figure 2).
 |
 |
 |
 |
 |
Molecular orbitals
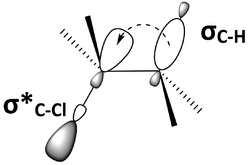
An important factor in the antiperiplanar conformer is the interaction between molecular orbitals. Anti-periplanar geometry will put a bonding orbital and an anti-bonding orbital approximately parallel to each other, or syn-periplanar. Figure 6 is another representation of 2-chloro-2,3-dimethylbutane (Figure 5), showing the C–H bonding orbital, σC–H, and the C–Cl anti-bonding orbital, σ*C–Cl, syn-periplanar. The parallel orbitals can overlap and become involved in hyperconjugation. If the bonding orbital is an electron donor and the anti-bonding orbital is an electron acceptor, then the bonding orbital will be able to donate electronegativity into the anti-bonding orbital. This filled-to-unfilled donor-acceptor interaction has an overall stabilizing effect on the molecule. However, donation from a bonding orbital into an anti-bonding orbital will also result in the weakening of both of those bonds. In Figure 6, 2-chloro-2,3-dimethylbutane is stabilized through hyperconjugation from electron donation from σC-H into σ*C-Cl, but both C–H and C–Cl bonds are weakened. A molecular orbital diagram shows that the mixing of σC–H and σ*C–Cl in 2-chloro-2,3-dimethylbutane lowers the energy of both the orbitals (Figure 7).
Examples of anti-periplanar geometry in mechanisms
E2 mechanism
A bimolecular elimination reaction will occur in a molecule where the breaking carbon-hydrogen bond and the leaving group are anti-periplanar[4][5][6][7] (Figure 8). This geometry is preferred because it aligns σC-H and σ*C-X orbitals.[8][9] Figure 9 shows the σC-H orbital and the σ*C-X orbital parallel to each other, allowing the σC-H orbital to donate into the σ*C-X anti-bonding orbital through hyperconjugation. This serves to weaken C-H and C-X bond, both of which are broken in an E2 reaction. It also sets up the molecule to more easily move its σC-H electrons into a πC-C orbital (Figure 10).
Pinacol rearrangement

In the pinacol rearrangement, a methyl group is found anti-periplanar to an activated alcohol functional group.[10][11] This places the σC–C orbital of the methyl group parallel with the σ*C–O orbital of the activated alcohol. Before the activated alcohol leaves as H2O the methyl bonding orbital donates into the C–O antibonding orbital, weakening both bonds. This hyperconjugation facilitates the 1,2-methyl shift that occurs to remove water. See Figure 11 for the mechanism.
History, etymology, and misuse
The term anti-periplanar was first coined by Klyne and Prelog in their work entitled "Description of steric relationships across single bonds", published in 1960.[12] ‘Anti’ refers to the two functional groups lying on opposite sides of the plane of the bond. ‘Peri’ comes from the Greek word for ‘near’ and so periplanar means “approximately planar”.[13] In their article “Periplanar or Coplanar?” Kane and Hersh point out that many organic textbooks use anti-periplanar to mean completely anti-planar, or anti-coplanar, which is technically incorrect.[14]
References
- ↑ Eliel, Ernest; Wilen, Samuel; Mander, Lewis (September 1994). Stereochemistry of Organic Compounds. New York: Wiley-Scientific. ISBN 978-0-471-01670-0.
- ↑ Kane, Saul; Hersh, William (1 October 2000). "Periplanar or Coplanar?". Journal of Chemical Education 77 (10): 1366. doi:10.1021/ed077p1366. Bibcode: 2000JChEd..77.1366K.
- ↑ Wikipedia, Dreamtheater at English (9 August 2012), English: An illustration of the syn/anti peri/clinal nomenclature of molecular torsional conformations. To be used on the page Alkane stereochemistry., https://commons.wikimedia.org/wiki/File:Synantipericlinal.svg, retrieved 2017-03-17
- ↑ Wade, Leroy (6 January 2012). Organic Chemistry (8th ed.). Pearson. pp. 267–268. ISBN 978-0321768414. https://archive.org/details/organicchemistry00wade_531.
- ↑ Carey, Francis; Sundberg, Richard (27 May 2008). Advanced Organic Chemistry: Part A: Structure and Mechanisms (5th ed.). Springer. pp. 558–563. ISBN 978-0387683461. https://archive.org/details/advancedorganicc00care_636.
- ↑ Deslongchamps, Ghislain; Deslongchamps, Pierre (12 May 2011). "Bent bonds, the antiperiplanar hypothesis and the theory of resonance. A simple model to understand reactivity in organic chemistry". Organic & Biomolecular Chemistry 9 (15): 5321–5333. doi:10.1039/C1OB05393K. PMID 21687842.
- ↑ Hunt, Ian; Spinney, Rick. "Chapter 5: Structure and Preparation of Alkenes. Elimination Reactions". http://www.chem.ucalgary.ca/courses/351/Carey5th/Ch05/ch5-6-1.html. Retrieved 13 March 2017.
- ↑ Anslyn, Eric; Dougherty, Dennis (15 July 2005). Modern Physical Organic Chemistry. University Science. pp. 590–592. ISBN 978-1891389313. https://archive.org/details/modernphysicalor00ansl.
- ↑ Rzepa, Henry (2012-02-04). "An orbtial analysis of the stereochemistry of the E2 elimination reaction". http://www.ch.imperial.ac.uk/rzepa/blog/?p=6205. Retrieved 13 March 2017.
- ↑ Anslyn, Eric; Dougherty, Dennis (15 July 2005). Modern Physical Organic Chemistry. University Science. pp. 676–677. ISBN 978-1891389313. https://archive.org/details/modernphysicalor00ansl.
- ↑ Carey, Francis; Sundberg, Richard (30 December 2010). Advanced Organic Chemistry: Part B: Reactions and Synthesis (5th ed.). Springer. pp. 883–886. ISBN 978-0387683546. https://archive.org/details/advancedorganicc00care_201.
- ↑ Klyne, William; Prelog, Vladimir (1 December 1960). "Description of steric relationships across single bonds". Experientia 16 (12): 521–523. doi:10.1007/BF02158433.
- ↑ Kane, Saul; Hersh, William (1 October 2000). "Periplanar or Coplanar?". Journal of Chemical Education 77 (10): 1366. doi:10.1021/ed077p1366. Bibcode: 2000JChEd..77.1366K.
- ↑ Kane, Saul; Hersh, William (1 October 2000). "Periplanar or Coplanar?". Journal of Chemical Education 77 (10): 1366. doi:10.1021/ed077p1366. Bibcode: 2000JChEd..77.1366K.
 |