Chemistry:Arecatannin
From HandWiki
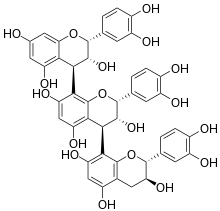 Chemical structure of arecatannin A1.
| |
| Names | |
|---|---|
| IUPAC name
(2R,3R,4S)-2-(3,4-dihydroxyphenyl)-4-[(2R,3S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-8-yl]-8-[(2R,3R,4R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C45H38O18 | |
| Molar mass | 866.77 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
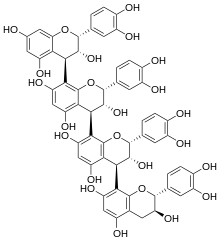 Chemical structure of arecatannin A2.
| |
| Names | |
|---|---|
| IUPAC name
(2R,3R,4R)-2-(3,4-dihydroxyphenyl)-4-[(2R,3R,4S)-2-(3,4-dihydroxyphenyl)-4-[(2R,3S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-8-yl]-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-8-yl]-8-[(2R,3R,4R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C60H50O24 | |
| Molar mass | 1155.03 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
 Chemical structure of arecatannin A3.
| |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C75H62O30 | |
| Molar mass | 1443.28 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
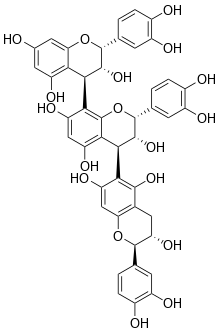 Chemical structure of arecatannin B1.
| |
| Names | |
|---|---|
| IUPAC name
(2R,3R,4S)-2-(3,4-dihydroxyphenyl)-4-[(2R,3S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-6-yl]-8-[(2R,3R,4R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C45H38O18 | |
| Molar mass | 866.77 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
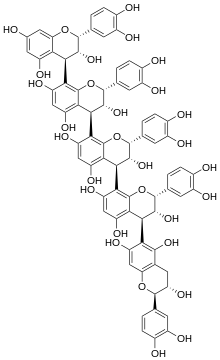 Chemical structure of arecatannin B2.
| |
| Names | |
|---|---|
| IUPAC name
(2R,3R,4R)-2-(3,4-dihydroxyphenyl)-4-[(2R,3R,4R)-2-(3,4-dihydroxyphenyl)-4-[(2R,3R,4S)-2-(3,4-dihydroxyphenyl)-4-[(2R,3S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-6-yl]-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-8-yl]-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-8-yl]-8-[(2R,3R,4R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C75H62O30 | |
| Molar mass | 1443.28 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
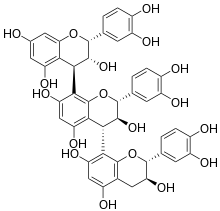 Chemical structure of arecatannin C1.
| |
| Names | |
|---|---|
| IUPAC name
(2R,3S,4R)-2-(3,4-dihydroxyphenyl)-4-[(2R,3S)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-8-yl]-8-[(2R,3R,4R)-2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-3,4-dihydro-2H-chromen-4-yl]-3,4-dihydro-2H-chromene-3,5,7-triol
| |
Other names
| |
| Identifiers | |
3D model (JSmol)
|
|
| KEGG | |
PubChem CID
|
|
| |
| |
| Properties | |
| C45H38O18 | |
| Molar mass | 866.77 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Short description: Group of chemical compounds
Arecatannins are a class of condensed tannins in the sub-class procyanidins contained in the seeds of Areca catechu also called betel nut.[1] The arecatannin-type natural products from Ceylonese cassia bark and Areca seed are examples of polyphenols by both current definitions, and fit the distinct definition of a polymeric phenol as well.[2]
Known molecules
The following six known arecatannins have been detected in A. catechu seeds.[3][4]
- Arecatannin A1
- Arecatannin A2
- Arecatannin A3
- Arecatannin B1
- Arecatannin B2
- Arecatannin C1
References
- ↑ Screening of various plant extracts used in ayurvedic medicine for inhibitory effects on human immunodeficiency virus type 1 (HIV-1) protease. Ines Tomoco Kusumoto, Takeshi Nakabayashi1, Hiroaki Kida, Hirotsugu Miyashiro, Masao Hattori, Tsuneo Namba and Kunitada Shimotohno, Phytotherapy Research, Volume 9, Issue 3, May 1995, pp. 180–184, doi:10.1002/ptr.2650090305
- ↑ "Isolation and structure elucidation of tannins. G. Nonaka, Pure & Appl. Chem.,Vol. 61, No. 3, pp. 357–360, 1989.". http://www.iupac.org/publications/pac/1989/pdf/6103x0357.pdf.
- ↑ "KEGG DRUG: Areca". https://www.genome.jp/dbget-bin/www_bget?dr:D06782.
- ↑ Peng, Wei; Liu, Yu-Jie; Wu, Na; Sun, Tao; He, Xiao-Yan; Gao, Yong-Xiang; Wu, Chun-Jie (April 2015). "Areca catechu L. (Arecaceae): A review of its traditional uses, botany, phytochemistry, pharmacology and toxicology". Journal of Ethnopharmacology 164: 340–356. doi:10.1016/j.jep.2015.02.010.
 |

