Chemistry:Arsenic dioxide
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
Arsenic(III,V) dioxide
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| AsO2 (As2O4) | |
| Molar mass | 106.920 g/mol |
| Hazards | |
| NIOSH (US health exposure limits): | |
PEL (Permissible)
|
[1910.1018] TWA 0.010 mg/m3[1] |
REL (Recommended)
|
Ca C 0.002 mg/m3 [15-minute][1] |
IDLH (Immediate danger)
|
Ca [5 mg/m3 (as As)][1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
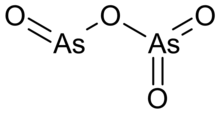
Arsenic dioxide is an inorganic compound with the chemical formula As2O4, containing As(III) and As(V), AsIIIAsVO4.[2]
Synthesis
It can be synthesized in an autoclave via the following reaction:
- 2 As2O3 + O2 → 2 As2O4
Structure
It adopts a layer structure, and the coordination geometry of As(III) is triangular pyramid while As(V) is tetrahedral.[2]
References
- ↑ 1.0 1.1 1.2 NIOSH Pocket Guide to Chemical Hazards. "#0038". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0038.html.
- ↑ 2.0 2.1 Jones, P. G.; Beesk, W.; Sheldrick, G. M.; Sharman, E. (14 February 1980). "Arsenic dioxide". Acta Crystallographica Section B 36 (2): 439–440. doi:10.1107/S0567740880003433.

