Chemistry:Aureoverticillactam
From HandWiki
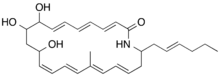
| |
| Names | |
|---|---|
| IUPAC name
(3E,5E,7E,13Z,15E,17E,19E)-22-[(E)-Hex-2-enyl]-9,10,12-trihydroxy-17-methyl-1-azacyclodocosa-3,5,7,13,15,17,19-heptaen-2-one[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| |
| Properties | |
| C28H39NO4 | |
| Molar mass | 453.623 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Aureoverticillactam is an antifungal macrocyclic lactam with the molecular formula C28H39NO4 which is produced by the marine bacterium Streptomyces aureoverticillatus.[3][1][4][5][6] Aureoverticillactam has also cytotoxic activity.[7][8]
References
- ↑ 1.0 1.1 "Aureoverticillactam" (in en). Pubchem.ncbi.NLM.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/Aureoverticillactam#section=3D-Conformer.
- ↑ (in en) Natural Products Atlas | Compounds. https://www.npatlas.org/explore/compounds/NPA011274.
- ↑ Blunt, John W.; Munro, Murray H. G. (19 September 2007) (in en). Dictionary of Marine Natural Products with CD-ROM. CRC Press. p. 157. ISBN 978-0-8493-8217-8.
- ↑ Mitchell, Scott S.; Nicholson, Benjamin; Teisan, Sy; Lam, Kin S.; Potts, Barbara C. M. (1 August 2004). "Aureoverticillactam, a Novel 22-Atom Macrocyclic Lactam from the Marine Actinomycete Streptomyces aureoverticillatus". Journal of Natural Products 67 (8): 1400–1402. doi:10.1021/np049970g. PMID 15332863.
- ↑ Communications, EBCONT. "Aureoverticillactam". Roempp.thieme.de.
- ↑ Wang, Lan-Ying; Zhang, Yun-Fei; Yang, De-You; Zhang, Shu-Jing; Han, Dan-Dan; Luo, Yan-Ping (2021). "Aureoverticillactam, a Potent Antifungal Macrocyclic Lactam from Streptomyces aureoverticillatus HN6, Generates Calcium Dyshomeostasis-Induced Cell Apoptosis via the Phospholipase C Pathway in Fusarium oxysporum f. sp. cubense Race 4" (in English). Phytopathology 111 (11): 2010–2022. doi:10.1094/PHYTO-12-20-0543-R. ISSN 0031-949X. PMID 33900117.
- ↑ Kim, Se-Kwon (14 March 2012) (in en). Marine Medicinal Foods: Implications and Applications: Animals and Microbes. Academic Press. p. 398. ISBN 978-0-12-416003-3.
- ↑ (in en) Studies in Natural Products Chemistry. Elsevier. 24 July 2008. p. 240. ISBN 978-0-08-056984-0.
Further reading
- Kim, Se-Kwon (27 November 2014) (in en). Handbook of Anticancer Drugs from Marine Origin. Springer. p. 245. ISBN 978-3-319-07145-9.
- Patra, Jayanta Kumar; Shukla, Amritesh C.; Das, Gitishree (30 March 2020) (in en). Advances in Pharmaceutical Biotechnology: Recent Progress and Future Applications. Springer Nature. p. 343. ISBN 978-981-15-2195-9.
 |

