Chemistry:Azeliragon
From HandWiki
| |
| Names | |
|---|---|
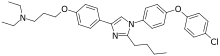
| IUPAC name
3-[4-[2-butyl-1-[4-(4-chlorophenoxy)phenyl]imidazol-4-yl]phenoxy]-N,N-diethylpropan-1-amine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C32H38ClN3O2 | |
| Molar mass | 532.13 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Azeliragon (TTP488 or PF-04494700) is a small-molecule RAGE inhibitor. It is developed by vTv Therapeutics for various cancers, including triple-negative breast cancer,[1][2] pancreatic cancer.[3]
The chemical reached Phase III trials in slowing cognitive deterioration in early stage Alzheimer's disease patients.[4][5][6] It was also tested in people with diabetic neuropathy[7] and animal models of graft-vs-host disease.[8]
References
- ↑ Magna, Melinda; Hwang, Gyong Ha; McIntosh, Alec; Drews-Elger, Katherine; Takabatake, Masaru; Ikeda, Adam; Mera, Barbara J.; Kwak, Taekyoung et al. (13 July 2023). "RAGE inhibitor TTP488 (Azeliragon) suppresses metastasis in triple-negative breast cancer" (in en). npj Breast Cancer 9 (1): 59. doi:10.1038/s41523-023-00564-9. ISSN 2374-4677. PMID 37443146.
- ↑ Xie, Jizhao; Xu, Huanji; Wu, Xinduo; Xie, Yunfeng; Lu, Xiuhong; Wang, Lisheng (December 2021). "Design, synthesis and anti-TNBC activity of Azeliragon triazole analogues". Bioorganic & Medicinal Chemistry Letters 54: 128444. doi:10.1016/j.bmcl.2021.128444. PMID 34763082.
- ↑ Kong, Weikang; Zhu, Lingxia; Li, Tian; Chen, Jiao; Fan, Bo; Ji, Wenjing; Zhang, Chunli; Cai, Xueting et al. (June 2023). "Azeliragon inhibits PAK1 and enhances the therapeutic efficacy of AKT inhibitors in pancreatic cancer". European Journal of Pharmacology 948: 175703. doi:10.1016/j.ejphar.2023.175703. PMID 37028543.
- ↑ Burstein, A.H.; Sabbagh, M.; Andrews, R.; Valcarce, C.; Dunn, I.; Altstiel, L. (2018). "DEVELOPMENT OF AZELIRAGON, AN ORAL SMALL MOLECULE ANTAGONIST OF THE RECEPTOR FOR ADVANCED GLYCATION ENDPRODUCTS, FOR THE POTENTIAL SLOWING OF LOSS OF COGNITION IN MILD ALZHEIMER'S DISEASE". The Journal of Prevention of Alzheimer's Disease 5 (2): 149–154. doi:10.14283/jpad.2018.18. PMID 29616709.
- ↑ Yang, Lijuan; Liu, Yepei; Wang, Yuanyuan; Li, Junsheng; Liu, Na (2021). "Azeliragon ameliorates Alzheimer's disease via the Janus tyrosine kinase and signal transducer and activator of transcription signaling pathway". Clinics 76: e2348. doi:10.6061/clinics/2021/e2348. PMID 33681944.
- ↑ Burstein, Aaron H.; Dunn, Imogene; Gooch, Ann M.; Valcarce, Carmen (December 2020). "Effects of azeliragon on ADAS‐cog and CDR domains and individual items in patients with mild Alzheimer's disease and type 2 diabetes (T2D): Human/Human trials: Other". Alzheimer's & Dementia 16 (S9). doi:10.1002/alz.041198.
- ↑ Ma, Simeng; Nakamura, Yoki; Hisaoka-Nakashima, Kazue; Morioka, Norimitsu (February 2023). "Blockade of receptor for advanced glycation end-products with azeliragon ameliorates streptozotocin-induced diabetic neuropathy". Neurochemistry International 163: 105470. doi:10.1016/j.neuint.2022.105470. PMID 36581174.
- ↑ Joshi, Aditi A.; Wu, Ying; Deng, Songyan; Preston-Hurlburt, Paula; Forbes, Josephine M.; Herold, Kevan C. (December 2022). "RAGE antagonism with azeliragon improves xenograft rejection by T cells in humanized mice.". Clinical Immunology 245: 109165. doi:10.1016/j.clim.2022.109165. PMID 36257528.
 |

