Chemistry:Azinomycin B
From HandWiki
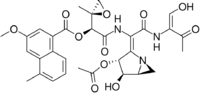
| |
| Names | |
|---|---|
| IUPAC name
(1S)-2-{[(1E)-1-[(3R,4R,5S)-3-Acetoxy-4-hydroxy-1-azabicyclo[3.1.0]hex-2-ylidene]-2-{[(1Z)-1-hydroxy-3-oxo-1-buten-2-yl]amino}-2-oxoethyl]amino}-1-[(2S)-2-methyl-2-oxiranyl]-2-oxoethyl 3-methoxy-5-methyl-1-naphthoate
| |
| Other names
Carzinophilin A; Carzinophillin A
| |
| Identifiers | |
3D model (JSmol)
|
|
| 9537192 | |
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C31H33N3O11 | |
| Molar mass | 623.615 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Azinomycin B is a natural product that contains densely assembled functionalities with potent antitumor activity.[1] It is isolated from Streptomyces sahachiroi[2] which is reisolated from S. griseofuscus along with its analog azinomycin A.[3] Azinomycin B can bind within the major groove of DNA and forms covalent interstrand crosslinks (ISCs) with the purine bases.[4][5][6][7] The DNA alkylation and crosslinking by azinomycin B suggests its potent antitumor activity.[8]
Biosynthesis
The biosynthesis of azinomycin B includes a type 1 polyketide synthase and several nonribosomal peptide synthetases.[9]

References
- ↑ Zhao, Qunfei, Qingli He, Wei Ding, Mancheng Tang, Qianjin Kang, Yi Yu, Wei Deng, Qi Zhang, Jie Fang, Gongli Tang, and Wen Liu. "Characterization of the Azinomycin B Biosynthetic Gene Cluster Revealing a Different Iterative Type I Polyketide Synthase for Naphthoate Biosynthesis." Chemistry & Biology 15.7 (2008): 693-705. Web.
- ↑ Hata, T., Koga, F., Sano, Y., Kanamori, K., Matsumae, A., Sunagawa, R., Hoshi, T., Shima, T., Ito, S., and Tomizawa, S. (1954). Carzinophilin, a new tumor inhibitory substance produced by Streptomyces. I. J. Antibiot. 7A, 107–112.
- ↑ Yokoi, K., Nagaoka, K., and Nakashima, T. (1986). Azinomycins A and B, new antitumor antibiotics. II. Chemical structures. Chem. Pharm. Bull. (Tokyo) 34, 4554–4561.
- ↑ Armstrong, R.W., Salvati, M.E., and Nguyen, M. (1992). Novel interstrand cross-links induced by the antitumor antibiotic carzinophillin/azinomycin B. J. Am. Chem. Soc. 114, 3144–3145.
- ↑ Hartley, J.A., Hazrati, A., Kelland, L.R., Khanim, R., Shipman, M., Suzenet, F., and Walker, L.F. (2000). A synthetic azinomycin analogue with demonstrated DNA cross-linking activity: Insights into the mechanism of action of this class of antitumor agent. Angew. Chem. Int. Ed. Engl. 39, 3467–3470.
- ↑ Coleman, R.S., Perez, R.J., Burk, C.H., and Navarro, A. (2002). Studies on the mechanism of action of azinomycin B: definition of regioselectivity and sequence selectivity of DNA cross-link formation and clarification of the role of the naphthoate. J. Am. Chem. Soc. 124, 13008–13017.
- ↑ LePla, R.C., Landreau, C.A.S., Shipman, M., and Jones, G.D. (2005). On the origin of the DNA sequence selectivity of the azinomycins. Org. Biomol. Chem. 3, 1174–1175.
- ↑ Kelly, G.T., Liu, C., Smith, R., III, Coleman, R.S., and Watanabe, C.M.H. (2006). Cellular effects induced by the antitumor agent azinomycin B. Chem. Biol. 13, 485–492.
- ↑ Zhao, Qunfei, Qingli He, Wei Ding, Mancheng Tang, Qianjin Kang, Yi Yu, Wei Deng, Qi Zhang, Jie Fang, Gongli Tang, and Wen Liu. "Characterization of the Azinomycin B Biosynthetic Gene Cluster Revealing a Different Iterative Type I Polyketide Synthase for Naphthoate Biosynthesis." Chemistry & Biology 15.7 (2008): 693-705. Web.
 |
