Chemistry:Azirinomycin
From HandWiki
| |
| Names | |
|---|---|
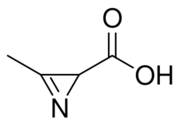
| IUPAC name
3-Methyl-2H-azirine-2-carboxylic acid[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
PubChem CID
|
|
| |
| |
| Properties | |
| C4H5NO2 | |
| Molar mass | 99.089 g·mol−1 |
| Related compounds | |
Related compounds
|
Azirine Motualevic acid F |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
Azirinomycin is an antibiotic azirine derivative with the molecular formula C4H5NO2 which is produced by the bacterium Streptomyces aureus.[1][2][3] Azirinomycin was first isolated in 1971.[4] Azirinomycin is toxic and therefore it cannot not be used in human medicine.[4]
References
- ↑ 1.0 1.1 "3-Methyl-2H-azirine-2-carboxylic acid" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/35931#section=3D-Conformer.
- ↑ Communications, EBCONT. "Azirinomycin". https://roempp.thieme.de/lexicon/RD-01-04135.
- ↑ Stapley, Edward O.; Hendlin, David; Jackson, Marion; Miller, A. Kathrine; Hernandez, Sebastian; Mata, Justo M. (25 January 1971). "Azirinomycin. I" (in en). The Journal of Antibiotics 24 (1): 42–47. doi:10.7164/antibiotics.24.42. ISSN 0021-8820.
- ↑ 4.0 4.1 Diana, Patrizia; Cirrincione, Girolamo (30 January 2015) (in en). Biosynthesis of Heterocycles: From Isolation to Gene Cluster. John Wiley & Sons. p. 101. ISBN 978-1-118-96042-4.
Further reading
- Miller, TW; Tristram, EW; Wolf, FJ (January 1971). "Azirinomycin. II. Isolation and chemical characterization as 3-methyl-2(2H) azirinecarboxylic acid.". The Journal of Antibiotics 24 (1): 48–50. doi:10.7164/antibiotics.24.48. PMID 5541332.
- Stapley, EO; Hendlin, D; Jackson, M; Miller, AK; Hernandez, S; Mata, JM (January 1971). "Azirinomycin. I. Microbial production and biological characteristics.". The Journal of Antibiotics 24 (1): 42–7. doi:10.7164/antibiotics.24.42. PMID 5541331.
- Williams, R. M. (22 October 2013) (in en). Synthesis of Optically Active Alpha-Amino Acids. Elsevier. p. 126. ISBN 978-1-4832-9295-3.
- Carreira, Erick M. (14 May 2014) (in en). Science of Synthesis Knowledge Updates 2011 Vol. 3. Georg Thieme Verlag. p. 137. ISBN 978-3-13-178751-4.
 |
