Chemistry:Bactobolin
From HandWiki
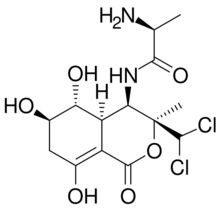
| |
| Names | |
|---|---|
| IUPAC name
(2S)-N-[(3S,4R,4aR,5R,6R)-3-(Dichloromethyl)-5,6,8-trihydroxy-3-methyl-1-oxo-4a,5,6,7-tetrahydro-4H-isochromen-4-yl]-2-aminopropanamide[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C14H20Cl2N2O6 | |
| Molar mass | 383.22 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bactobolin is a cytotoxic, polyketide-peptide and antitumor antibiotic with the molecular formula C14H20Cl2N2O6.[2][3][4][5] Bactobolin was discovered in 1979.[6]
References
- ↑ 1.0 1.1 "Bactobolin" (in en). Pubchem.ncbi.NLM.nih.gov. https://pubchem.ncbi.nlm.nih.gov/compound/Bactobolin.
- ↑ Greenberg, E. Peter; Chandler, Josephine R.; Seyedsayamdost, Mohammad R. (27 March 2020). "The Chemistry and Biology of Bactobolin: A 10-Year Collaboration with Natural Product Chemist Extraordinaire Jon Clardy". Journal of Natural Products 83 (3): 738–743. doi:10.1021/acs.jnatprod.9b01237. PMID 32105069.
- ↑ Weinreb, Steven M. (1995). "Total synthesis of the microbial antitumor antibiotics actinobolin and bactobolin". Studies in Natural Products Chemistry 16: 3–25. doi:10.1016/S1572-5995(06)80051-7. ISBN 9780444822642.
- ↑ Munakata, Tomohiko; Ikeda, Yoshifumi; Matsuki, Hideo; Isagai, Katsuyoshi (May 1983). "Cultural Conditions and Strain Improvement for Production of Bactobolin". Agricultural and Biological Chemistry 47 (5): 929–934. doi:10.1080/00021369.1983.10865772.
- ↑ Franco, Carlos M.; Belda, Beatriz Vázquez (2 December 2020) (in en). Natural Compounds as Antimicrobial Agents. MDPI. p. 134. ISBN 978-3-03936-048-2.
- ↑ Reinhoudt, D. N.; Connors, T. A.; Pinedo, H. M.; Poll, K. W. van de (6 December 2012) (in en). Structure-Activity Relationships of Anti-Tumour Agents. Springer Science & Business Media. p. 270. ISBN 978-94-009-6798-4.
Further reading
- Weinreb, S. M.; Garigipati, R. S. (1 January 1989). "Design of an efficient strategy for total synthesis of the microbial metabolite (-)-bactobolin". Pure and Applied Chemistry 61 (3): 435–438. doi:10.1351/pac198961030435.
- Lukacs, Gabor; Ohno, Masaji (6 December 2012) (in en). Recent Progress in the Chemical Synthesis of Antibiotics. Springer Science & Business Media. p. 286. ISBN 978-3-642-75617-7.
- Kocienski, Philip J. (14 May 2014) (in en). Protecting Groups, 3rd Edition 2005. Georg Thieme Verlag. p. 554. ISBN 978-3-13-179153-5.
 |

