Chemistry:Benzimidazolinone
| |
| Names | |
|---|---|
| IUPAC name
2-Benzimidazolinone
| |
| Other names
1,3-Dihydro-2H-benzimidazol-2-one
2-Hydroxybenzimidazole N,N′-(1,2-Phenylene)urea | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C7H6N2O | |
| Molar mass | 134.138 g·mol−1 |
| Appearance | white solid |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H302 | |
| P264, P270, P301+317Script error: No such module "Preview warning".Category:GHS errors, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
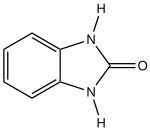
Benzimidazolinone is an organic compound with the formula C
6H
4(NH)
2CO. Also classified as a heterocyclic compound it is a bicyclic urea. It is a tautomer of 2-hydroxybenzimidazole.
Synthesis, structure, applications
The parent compound is prepared by the carbonylation of 1,2-diaminobenzene. The carbonylation can be effected with carbonyldiimidazole.[2] Like other ureas, it engages in hydrogen bonding, yielding supramolecular structures. Otherwise, the compound is of little interest.
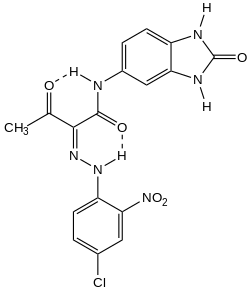
Substituted 2-benzimidazolinones are commercial dyes and pigments. For example 4-amino-2-benzimidazolinone condenses with diketene to give the acetoacetanilide, which undergoes diazo coupling with various aryldiazonium salts. In this way pigment orange 36 and pigment yellow 154 are produced. These pigments are used in paints and plastics.[3]
The drug domperidone is a derivative of benzimidazolinone.
References
- ↑ "2-Hydroxybenzimidazole" (in en). https://pubchem.ncbi.nlm.nih.gov/compound/11985#section=Safety-and-Hazards.
- ↑ Schwiebert, Kathryn E.; Chin, Donovan N.; MacDonald, John C.; Whitesides, George M. (1996). "Engineering the Solid State with 2-Benzimidazolones". Journal of the American Chemical Society 118 (17): 4018–4029. doi:10.1021/ja952836l.
- ↑ Jaffe, Edward E. (2004). "Pigments, Organic". Kirk-Othmer Encyclopedia of Chemical Technology. doi:10.1002/0471238961.151807011001060605.a01.pub2. ISBN 978-0-471-48494-3.
 |