Chemistry:Berkelium(III) bromide
From HandWiki
| |
| Identifiers | |
|---|---|
3D model (JSmol)
|
|
| ChemSpider | |
| |
| |
| Properties | |
| BkBr3 | |
| Molar mass | 487 g·mol−1 |
| Appearance | yellow-green crystals[1] |
| Related compounds | |
Other anions
|
Berkelium fluoride Berkelilum chloride Berkelium iodide |
Other cations
|
Curium bromide Californium bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Berkelium bromide is a bromide of berkelium, with the chemical formula BkBr3.
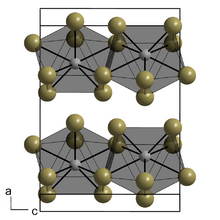
Structure
Berkelium bromide has a PuBr3 structure at low temperature and is in the orthorhombic crystal system, with lattice parameters a = 403 pm, b = 1271 pm and c = 912 pm.[2] At high temperature, berkelium bromide has an AlCl3 structure and a monoclinic crystal system with lattice parameters a = 723 pm, b = 1253 pm, c = 683 pm and β = 110.6°.[2][3][4][5]
References
- ↑ A. F. Holleman, E. Wiberg, N. Wiberg: Lehrbuch der Anorganischen Chemie. 102. Auflage. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1, S. 1969.
- ↑ 2.0 2.1 Burns, John H.; Peterson, J.R.; Stevenson, J.N. (Mar 1975). "Crystallographic studies of some transuranic trihalides: 239PuCl3, 244CmBr3, 249BkBr3 and 249CfBr3" (in en). Journal of Inorganic and Nuclear Chemistry 37 (3): 743–749. doi:10.1016/0022-1902(75)80532-X. https://linkinghub.elsevier.com/retrieve/pii/002219027580532X.
- ↑ Young, J. P.; Haire, R. G.; Peterson, J. R.; Ensor, D. D.; Fellows, R. L. (Aug 1980). "Chemical consequences of radioactive decay. 1. Study of californium-249 ingrowth into crystalline berkelium-249 tribromide: a new crystalline phase of californium tribromide" (in en). Inorganic Chemistry 19 (8): 2209–2212. doi:10.1021/ic50210a003. ISSN 0020-1669. https://pubs.acs.org/doi/abs/10.1021/ic50210a003.
- ↑ Cohen, D.; Fried, S.; Siegel, S.; Tani, B. (May 1968). "The preparation and crystal structure of some berkelium compounds" (in en). Inorganic and Nuclear Chemistry Letters 4 (5): 257–260. doi:10.1016/0020-1650(68)80125-4. https://linkinghub.elsevier.com/retrieve/pii/0020165068801254.
- ↑ Peterson, J.R.; Hobart, D.E. (1984), "The Chemistry of Berkelium" (in en), Advances in Inorganic Chemistry (Elsevier) 28: pp. 29–72, doi:10.1016/s0898-8838(08)60204-4, ISBN 978-0-12-023628-2, https://linkinghub.elsevier.com/retrieve/pii/S0898883808602044, retrieved 2023-06-26
External reading
- Hobart, David E.; Peterson, Joseph R. (2006), Morss, Lester R.; Edelstein, Norman M.; Fuger, Jean, eds., "Berkelium" (in en), The Chemistry of the Actinide and Transactinide Elements (Dordrecht: Springer Netherlands): pp. 1444–1498, doi:10.1007/1-4020-3598-5_10, ISBN 978-1-4020-3555-5, http://link.springer.com/10.1007/1-4020-3598-5_10, retrieved 2023-06-26
 |

