Chemistry:Betulin
From HandWiki
| |
| Names | |
|---|---|
| IUPAC name
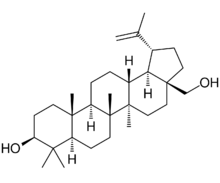
Lup-20(29)-ene-3β,28-diol
| |
| Systematic IUPAC name
(1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-3a-(Hydroxymethyl)-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)icosahydro-1H-cyclopenta[a]chrysen-9-ol | |
| Other names
Betulinol, betuline, betulol, betulinic alcohol, trochol
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| EC Number |
|
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C30H50O2 | |
| Molar mass | 442.728 g·mol−1 |
| Appearance | solid with needle-like crystals[1] |
| Melting point | 256 to 257 °C (493 to 495 °F; 529 to 530 K) |
| insoluble[1] | |
| Solubility | slightly soluble in ethanol and benzene; soluble in diethyl ether, ethyl acetate and ligroin[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Betulin is an abundant, naturally occurring triterpene. It is commonly isolated from the bark of birch trees. It forms up to 30% of the dry weight of silver birch bark.[2] It is also found in birch sap.[citation needed] Inonotus obliquus contains betulin.[3]
The compound in the bark gives the tree its white color which appears to protect the tree from mid-winter overheating by the sun. As a result, birches are some of the northernmost occurring deciduous trees.
History
Betulin was discovered in 1788 by German-Russian chemist Johann Tobias Lowitz.[4][5]
Chemistry
Chemically, betulin is a triterpenoid of lupane structure. It has a pentacyclic ring structure, and hydroxyl groups in positions C3 and C28.
See also
References
- ↑ 1.0 1.1 1.2 Haynes, William M.; Lide, David R.; Bruno, Thomas J. (2014). "3". CRC Handbook of Chemistry and Physics (95th ed.). Boca Raton, Florida: CRC Press. pp. 340. ISBN 9781482208689. OCLC 908078665. https://books.google.com/books?id=bNDMBQAAQBAJ&pg=SA7-PA.
- ↑ Green, Brian; Bentley, Michael D.; Chung, Bong Y.; Lynch, Nicholas G.; Jensen, Bruce L. (2007-12-01). "Isolation of Betulin and Rearrangement to Allobetulin. A Biomimetic Natural Product Synthesis" (in EN). Journal of Chemical Education 84 (12): 1985. doi:10.1021/ed084p1985. Bibcode: 2007JChEd..84.1985G.
- ↑ Gao, Yuan; Xu, Hongyu; Lu, Zhenming; Xu, Zhenghong (November 2009). "Quantitative determination of steroids in the fruiting bodies and submerged-cultured mycelia of Inonotus obliquus". Se Pu 27 (6): 745–749. ISSN 1000-8713. PMID 20352924.
- ↑ Lowitz, J. T. (1788). "Űber eine neue, fast benzoeartige substanz der briken". Crell's Chem. Ann. 1: 312–317.
- ↑ Król, Sylwia Katarzyna; Kiełbus, Michał; Rivero-Müller, Adolfo; Stepulak, Andrzej (2015). "Comprehensive Review on Betulin as a Potent Anticancer Agent". BioMed Research International 2015: 584189. doi:10.1155/2015/584189. PMID 25866796.
 |
