Chemistry:Bicyclo(1.1.1)pentane
From HandWiki
Short description: Chemical compound
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Bicyclo[1.1.1]pentane | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
PubChem CID
|
|||
| |||
| |||
| Properties | |||
| C5H8 | |||
| Molar mass | 68.119 g·mol−1 | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
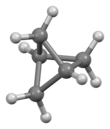
Bicyclo[1.1.1]pentane is an organic compound, the simplest member of the bicyclic bridged compounds family. It is a hydrocarbon with formula C5H8. The molecular structure consists of three rings of four carbon atoms each.
Bicyclo[1.1.1]pentane is a highly strained molecule.
Usage
Kenneth Wiberg and Frederick Walker used bicyclo[1.1.1]pentane to make [1.1.1]propellane.[1]
The bicyclo[1.1.1]pentane structure has been used as an unusual bioisostere for a phenyl ring.[2]
See also
References
- ↑ Wiberg, Kenneth B.; Walker, Frederick H. (1982). "[1.1.1]Propellane". J. Am. Chem. Soc. 104 (19): 5239–5240. doi:10.1021/ja00383a046.
- ↑ Stepan, Antonia F.; Subramanyam, Chakrapani; Efremov, Ivan V.; Dutra, Jason K.; O’Sullivan, Theresa J.; DiRico, Kenneth J.; McDonald, W. Scott; Won, Annie et al. (2012-04-12). "Application of the Bicyclo[1.1.1pentane Motif as a Nonclassical Phenyl Ring Bioisostere in the Design of a Potent and Orally Active γ-Secretase Inhibitor"]. Journal of Medicinal Chemistry 55 (7): 3414–3424. doi:10.1021/jm300094u. ISSN 0022-2623. PMID 22420884. https://figshare.com/articles/Application_of_the_Bicyclo_1_1_1_pentane_Motif_as_a_Nonclassical_Phenyl_Ring_Bioisostere_in_the_Design_of_a_Potent_and_Orally_Active_Secretase_Inhibitor/2531245.
 |