Chemistry:Bis(pentafluorophenyl)xenon
| |
| Names | |
|---|---|
| IUPAC name
bis(2,3,4,5,6-pentafluorophenyl)xenon
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
CompTox Dashboard (EPA)
|
|
| |
| |
| Properties | |
| C12F10Xe | |
| Molar mass | 465.409 g·mol−1 |
| Density | 2.447 g/cm3 (at 50 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
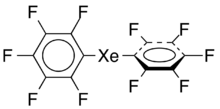
Bis(pentafluorophenyl)xenon is an unstable organic compound of xenon. It consists of two fluoronated phenyl rings connected to xenon.
Structure
Bis(pentafluorophenyl)xenon is a molecular substance. In the solid form it crystallises in the monoclinic system with space group P21/n.[1] The unit cell has four molecules with a=13.635 Å b=8.248 Å c=11.511 Å, β =102.624°. Unit cell volume is 1263.18 Å3.[2]
The molecules have carbon to xenon to carbon bonds in nearly a straight line (the bond angle is at least 175°). The carbon–xenon bond-lengths are 2.35 and 2.39 Å. The two pentafluorophenyl rings are twisted by 72° with respect to each other.[1]
Properties
Bis(pentafluorophenyl)xenon decomposes above −20 °C and can explode.[1]
Preparation
Bis(pentafluorophenyl)xenon is prepared in liquid dichloromethane at −40 °C.[1] It uses a tetramethyl ammonium fluoride catalyst.[3]
XeF2 + 2(CH3)3SiC6F5 → Xe(C6F5)2 + 2(CH3)3SiF
2C6F5XeF + Cd(C6F5)2 → Xe(C6F5)2 +CdF2
Reactions
Bis(pentafluorophenyl)xenon reacts with hydrogen fluoride to form pentafluorophenyl xenon fluoride C6F5XeF.[3] In acetonitrile solution bis(pentafluorophenyl)xenon decomposes to form C6F5-C6F5 (C12F10) and xenon.[3] But in dichloromethane solution the product is mostly pentafluorobenzene.[3]
Bis(pentafluorophenyl)xenon reacts with mercury to make Bis(pentafluorophenyl)mercury.[3]
It reacts with iodine to make pentafluoroiodobenzene. C6F5I.[3]
References
- ↑ 1.0 1.1 1.2 1.3 Bock, Harald; Hinz-Hübner, Dirk; Ruschewitz, Uwe; Naumann, Dieter (1 February 2002). "Structure of Bis(pentafluorophenyl)xenon, Xe(C6F5)2". Angewandte Chemie International Edition 41 (3): 448–450. doi:10.1002/1521-3773(20020201)41:3<448::AID-ANIE448>3.0.CO;2-W.
- ↑ Bock, H.; Hinz-Hubner, D.; Ruschewitz, U.; Naumann, D. (2002). CCDC 169453: Experimental Crystal Structure Determination. Cambridge Crystallographic Data Centre. doi:10.5517/cc5pb7d.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 Frohn, Hermann-Josef; Bardin, Vadim V. (November 2001). "Preparation and Reactivity of Compounds Containing a Carbon−Xenon Bond". Organometallics 20 (23): 4750–4762. doi:10.1021/om010490j.

