Chemistry:Bisbenzylisoquinoline alkaloids
Bisbenzylisoquinoline alkaloids are natural products found primarily in the plant families of the barberry family, the Menispermaceae, the Monimiaceae, and the buttercup family.[1]
Occurrence
More than 225 different bisbenzylisoquinoline alkaloids are known and have been isolated.[1]
Representative
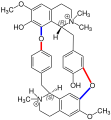
The bisbenzylisoquinoline alkaloids are considered the largest group within the isoquinoline alkaloids.[2] The best-known representative of this group is tubocurarine chloride. Other representatives include dauricin, oxyacanthin, tetrandrine, and tiliacorin.[3]
Structure
Bisbenzylisoquinoline alkaloids are characterized by their structure. Typically, they consist of two benzyl-tetrahydroquinoline units linked by ether groups, and occasionally by C-C bonds. Multiple ether bridges are often present.[1][4] These alkaloids can be categorized into three groups, using the nomenclature head for the 1,2,3,4-tetrahydroisoquinoline unit and tail for the 1-benzyl residue:
- head-head
- tail-tail-
- head-tail-linked bisbenzylisoquinoline alkaloids.[3]
Dauricine is the simplest representative with a tail-to-tail linkage. Oxyacanthine and Tetrandrin contain both head-head and tail-tail linkages, while Tiliacorine features one tail-tail linkage and two head-head linkages linking to a dibenzodioxin moiety. Tubocurarine chloride is characterized by two head-tail linkages of the tetrahydroisoquinoline units.[3] In the following structural formulae, the tail linkages are marked red and the head linkages are marked blue:
Uses
The alkaloids belonging to the bisbenzylisoquinoline group are generally toxic and exhibit curarizing effects. Oxyacanthine serves as a sympatholytic agent, an antagonist to epinephrine, and a vasodilator. Tubocurarine, a potent curarizing poison, stands as the oldest known muscle relaxant. South American indigenous populations have traditionally employed it as an arrow poison (see curare).[1]
Tetrandine, found as an ingredient in the Chinese medicine "han-fang-shi," possesses analgesic and antipyretic properties.[3]
References
- ↑ 1.0 1.1 1.2 1.3 Entry on Bisbenzylisoquinoline alkaloids. at: Römpp Online. Georg Thieme Verlag, retrieved {{{Datum}}}.
- ↑ Peter Nuhn (2006), Naturstoffchemie (4 ed.), Stuttgart: S.Hirzel Verlag, pp. 603f, ISBN 9783777613635
- ↑ 3.0 3.1 3.2 3.3 Eberhard Breitmaier (1997), Alkaloide, Wiesbaden: Springer Fachmedien, pp. 63f, ISBN 9783519035428
- ↑ H. Latscha, U. Kazmaier (2016), Chemie für Biologen (4 ed.), Berlin Heidelberg: Springer Spektrum, pp. 691, ISBN 9783662477830
 |