Biology:Curare
Curare (/kʊˈrɑːri/ or /kjʊˈrɑːri/; kuu-RAH-ree or kyuu-RAH-ree) is a common name for various alkaloid arrow poisons originating from plant extracts. Used as a paralyzing agent by indigenous peoples in Central and South America for hunting and for therapeutic purposes, curare only becomes active when it contaminates a wound or is introduced directly to the bloodstream; it is not active when ingested orally. These poisons cause weakness of the skeletal muscles and, when administered in a sufficient dose, eventual death by asphyxiation due to paralysis of the diaphragm. Curare is prepared by boiling the bark of one of the dozens of plant sources, leaving a dark, heavy paste that can be applied to arrow or dart heads. In medicine, curare has been used as a treatment for tetanus and strychnine poisoning and as a paralyzing agent for surgical procedures.
History
This section is missing information about use of curare by Central American people. (March 2014) |
The word 'curare' is derived from wurari, from the Carib language of the Macusi of Guyana.[1] It has its origins in the Carib phrase "mawa cure" meaning of the Mawa vine, scientifically known as Strychnos toxifera.[citation needed] Curare is also known among indigenous peoples as Ampi, Woorari, Woorara, Woorali, Wourali, Wouralia, Ourare, Ourari, Urare, Urari, and Uirary. The noun 'curare' is not to be confused with the Latin verb 'curare' ('to heal, cure, take care of').
Classification
In 1895 pharmacologist Rudolf Boehm sought to classify the various alkaloid poisons based on the containers used for their preparation. He believed curare could be categorized into three main types as seen below. However useful it appeared, it became rapidly outmoded. Richard Gill, a plant collector, found that the indigenous peoples began to use a variety of containers for their curare preparations, henceforth invalidating Boehm's basis of classification.[2]
- Tube or bamboo curare: Mainly composed of the toxin D-tubocurarine, this poison is found packed into hollow bamboo tubes derived from Chondrodendron and other genera in the Menispermaceae. According to their LD50 values, tube curare is thought to be the most toxic.
- Pot curare: Mainly composed of alkaloid components protocurarine (the active ingredient), protocurine (a weak toxicity), and protocuridine (non-toxic) from both Menispermaceae and Loganiaceae/Strychnaceae. This subtype is found originally packed in terra cotta pots.
- Calabash or gourd curare: Mainly composed of C toxiferine I, this poison was originally packed into hollow gourds from Loganiaceae/Strychnaceae alone.
Manske also observed in his 1955 The Alkaloids:
The results of the early [pre-1900] work were very inaccurate because of the complexity and variation of the composition of the mixtures of alkaloids involved ... these were impure, non-crystalline alkaloids ... Almost all curare preparations were and are complex mixtures, and many of the physiological actions attributed to the early curarizing preparations were undoubtedly due to impurities, particularly to other alkaloids present. The curare preparations are now considered to be of two main types, those from Chondrodendron or other members of the Menispermaceae family and those from Strychnos, a genus of the Loganiaceae [ now Strychnaceae ] family. Some preparations may contain alkaloids from both ... and the majority have other secondary ingredients.[2]
Hunting uses
Curare was used as a paralyzing poison by many South American indigenous people. Since it was too expensive to be used in warfare, curare was mainly used for hunting.[3] The prey was shot by arrows or blowgun darts dipped in curare, leading to asphyxiation owing to the inability of the victim's respiratory muscles to contract. In particular, the poison was used by the Kalinago, indigenous people of the Lesser Antilles in the Caribbean, on the tips of their arrows.[4] In addition, the Yagua people, indigenous to Colombia and northeastern Peru, commonly used these toxins via blowpipes to target prey 30 to 40 paces distant.[5]
Due to its popularity among the indigenous people as means of paralyzing prey, certain tribes would create monopolies from curare production.[3] Thus, curare became a symbol of wealth among the indigenous populations.
In 1596, Sir Walter Raleigh mentioned the arrow poison in his book Discovery of the Large, Rich, and Beautiful Empire of Guiana (which relates to his travels in Trinidad and Guayana), though the poison he described was possibly not curare.[6] In 1780, Abbe Felix Fontana discovered that it acted on the voluntary muscles rather than the nerves and the heart.[7] In 1832, Alexander von Humboldt gave the first western account of how the toxin was prepared from plants by Orinoco River natives.[8]

During 1811–1812, Sir Benjamin Collins Brody experimented with curare (woorara).[9][10] He was the first to show that curare does not kill the animal and the recovery is complete if the animal's respiration is maintained artificially. In 1825, Charles Waterton described a classical experiment in which he kept a curarized female donkey alive by artificial respiration with a bellows through a tracheostomy.[11] Waterton is also credited with bringing curare to Europe.[12] Robert Hermann Schomburgk, who was a trained botanist, identified the vine as one of the genus Strychnos and gave it the now accepted name Strychnos toxifera.[13]
Medical use
George Harley (1829–1896) showed in 1850 that curare (wourali) was effective for the treatment of tetanus and strychnine poisoning.[14][15] In 1857, Claude Bernard (1813–1878) published the results of his experiments in which he demonstrated that the mechanism of action of curare was a result of interference in the conduction of nerve impulses from the motor nerve to the skeletal muscle, and that this interference occurred at the neuromuscular junction.[16][17] From 1887, the Burroughs Wellcome catalogue listed under its 'Tabloids' brand name, 1⁄12 grain (5.4 mg) tablets of curare (price: 8 shillings) for use in preparing a solution for hypodermic injection. In 1914, Henry Hallett Dale (1875–1968) described the physiological actions of acetylcholine.[18] After 25 years, he showed that acetylcholine is responsible for neuromuscular transmission, which can be blocked by curare.[19]

The best known and historically most important toxin (because of its medical applications) is d-tubocurarine. It was isolated from the crude drug – from a museum sample of curare – in 1935 by Harold King of London, working in Sir Henry Dale's laboratory. King also established its chemical structure.[20][21] Pascual Scannone, a Venezuelan anesthesiologist[22] who trained and specialized in New York City, did extensive research on curare as a possible paralyzing agent for patients during surgical procedures. In 1942, he became the first person in Latin America to use curare during a medical procedure when he successfully performed a tracheal intubation in a patient to whom he administered curare for muscle paralysis at the El Algodonal Hospital in Caracas, Venezuela.[22]
After its introduction in 1942, curare/curare-derivatives became a widely used paralyzing agent during medical and surgical procedures.[citation needed] In medicine, curare has been superseded by a number of curare-like agents, such as pancuronium, which have a similar pharmacodynamic profile, but fewer side effects.[citation needed]
Chemical structure
The various components of curare are organic compounds classified as either isoquinoline or indole alkaloids. Tubocurarine is one of the major active components in the South American dart poison.[23] As an alkaloid, tubocurarine is a naturally occurring compound that consists of nitrogenous bases, although the chemical structure of alkaloids is highly variable.

Tubocurarine and C toxiferine consist of a cyclic system with quaternary ammonium ions. On the other hand, while acetylcholine does not contain a cyclic system, it does contain a quaternary ammonium ion. Because of this shared moiety, curare alkaloids can bind readily to the active site of receptors for acetylcholine (ACh) at the neuromuscular junction, blocking nerve impulses from being sent to the skeletal muscles, effectively paralyzing the muscles of the body.
Pharmacological properties
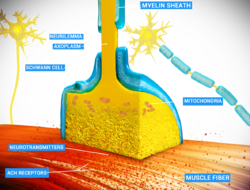
Curare is an example of a non-depolarizing muscle relaxant that blocks the nicotinic acetylcholine receptor (nAChR),[24] one of the two types of acetylcholine (ACh) receptors, at the neuromuscular junction. The main toxin of curare, d-tubocurarine, occupies the same position on the receptor as ACh with an equal or greater affinity, and elicits no response, making it a competitive antagonist. The antidote for curare poisoning is an acetylcholinesterase (AChE) inhibitor (anti-cholinesterase), such as physostigmine or neostigmine. By blocking ACh degradation, AChE inhibitors raise the amount of ACh in the neuromuscular junction; the accumulated ACh will then correct for the effect of the curare by activating the receptors not blocked by toxin at a higher rate.
The time of onset varies from within one minute (for tubocurarine in intravenous administration, penetrating a larger vein), to between 15 and 25 minutes (for intramuscular administration, where the substance is applied in muscle tissue).[24]
It is harmless if taken orally[25][26] because curare compounds are too large and highly charged to pass through the lining of the digestive tract to be absorbed into the blood. For this reason, people can safely eat curare-poisoned prey, and it has no effect on its flavor.[27]
Anesthesia
Isolated attempts to use curare during anesthesia date back to 1912 by Arthur Lawen of Leipzig,[28] but curare came to anesthesia via psychiatry (electroplexy). In 1939 Abram Elting Bennett used it to modify metrazol induced convulsive therapy.[29] Muscle relaxants are used in modern anesthesia for many reasons, such as providing optimal operating conditions and facilitating intubation of the trachea. Before muscle relaxants, anesthesiologists needed to use larger doses of the anesthetic agent, such as ether, chloroform or cyclopropane to achieve these aims. Such deep anesthesia risked killing patients who were elderly or had heart conditions.
The source of curare in the Amazon was first researched by Richard Evans Schultes in 1941. Since the 1930s, it was being used in hospitals as a muscle relaxant. He discovered that different types of curare called for as many as 15 ingredients, and in time helped to identify more than 70 species that produced the drug.
In the 1940s, it was used on a few occasions during surgery as it was mistakenly thought to be an analgesic or anesthetic. The patients reported feeling the full intensity of the pain though they were not able to do anything about it since they were essentially paralyzed.[30]
On January 23, 1942, Harold Griffith and Enid Johnson gave a synthetic preparation of curare (Intercostrin/Intocostrin) to a patient undergoing an appendectomy (to supplement conventional anesthesia). Safer curare derivatives, such as rocuronium and pancuronium, have superseded d-tubocurarine for anesthesia during surgery. When used with halothane d-tubocurarine can cause a profound fall in blood pressure in some patients as both the drugs are ganglion blockers.[31] However, it is safer to use d-tubocurarine with ether.
In 1954, an article was published by Beecher and Todd suggesting that the use of muscle relaxants (drugs similar to curare) increased death due to anesthesia nearly sixfold.[32] This was refuted in 1956.[33]
Modern anesthetists have at their disposal a variety of muscle relaxants for use in anesthesia. The ability to produce muscle relaxation irrespective of sedation has permitted anesthetists to adjust the two effects independently and on the fly to ensure that their patients are safely unconscious and sufficiently relaxed to permit surgery. The use of neuromuscular blocking drugs carries with it the risk of anesthesia awareness.
Plant sources
There are dozens of plants from which isoquinoline and indole alkaloids with curarizing effects can be isolated, and which were utilized by indigenous tribes of Central and South America for the production of arrow poisons. Among them are:
In family Menispermaceae:
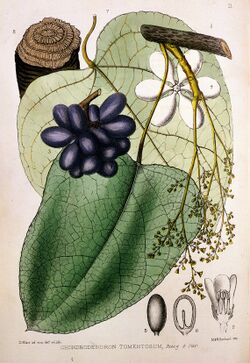
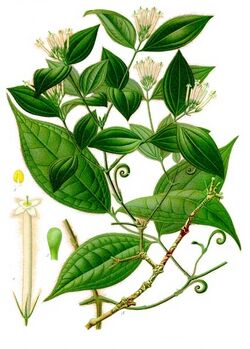
- Genus Chondrodendron notably C. tomentosum
- Genus Curarea, species C. toxicofera and C. tecunarum
- Genus Sciadotenia toxifera
- Genus Telitoxicum
- Genus Abuta
- Genus Caryomene
- Genus Anomospermum
- Genus Orthomene
- Genus Cissampelos, section L. (Cocculeae) of genus
Other families:
- several species of the genus Strychnos of family Loganiaceae including S. toxifera, S. guianensis, S. castelnaei, S. usambarensis
- a plant in the subfamily Aroideae of family Araceae called taja
- at least three members of the genus Artanthe of family Piperaceae
- Paullinia cururu in the family Sapindaceae[34]
Some plants in the family Aristolochiaceae have also been reported as sources.
Alkaloids with curare-like activity are present in plants of the fabaceous genus Erythrina.[2]
Toxicity
The toxicity of curare alkaloids in humans has not been established. Administration must be parenterally, as gastro-intestinal absorption is ineffective.
LD50 (mg/kg)
human: 0.735 est. (form and method of administration not indicated)
mouse: pot: 0.8–25; tubo: 5-10; calabash: 2–15.
Preparation
In 1807, Alexander von Humboldt provided the first eye-witness account of curare preparation.[3] A mixture of young bark scrapings of the Strychnos plant, other cleaned plant parts, and occasionally snake venom is boiled in water for two days. This liquid is then strained and evaporated to create a dark, heavy, viscid paste that would be tested for its potency later.[3] This curare paste was described to be very bitter in taste.
In 1938, Richard Gill and his expedition collected samples of processed curare and described its method of traditional preparation; one of the plant species used at that time was Chondrodendron tomentosum.[35]
Adjuvants
Various irritating herbs, stinging insects, poisonous worms, and various parts of amphibians and reptiles are added to the preparation. Some of these accelerate the onset of action or increase the toxicity; others prevent the wound from healing or blood from coagulating.
Diagnosis and management of curare poisoning
Curare poisoning can be indicated by typical signs of neuromuscular-blocking drugs such as paralysis including respiration but not directly affecting the heart.
Curare poisoning can be managed by artificial respiration such as mouth-to-mouth resuscitation. In a study of 29 army volunteers that were paralyzed with curare, artificial respiration managed to keep an oxygen saturation of always above 85%,[36] a level at which there is no evidence of altered state of consciousness.[37] Yet, curare poisoning mimics the total locked-in syndrome in that there is paralysis of every voluntarily controlled muscle in the body (including the eyes), making it practically impossible for the victim to confirm consciousness while paralyzed.[38]
Spontaneous breathing is resumed after the end of the duration of action of curare, which is generally between 30 minutes[39] and 8 hours,[40] depending on the variant of the toxin and dosage. Cardiac muscle is not directly affected by curare, but if more than four to six minutes[41] has passed since respiratory cessation the cardiac muscle may stop functioning by oxygen-deprivation, making cardiopulmonary resuscitation including chest compressions necessary.
Chemical antidote
Since tubocurarine and the other components of curare bind reversibly to the ACh receptors, treatment for curare poisoning involves adding an acetylcholinesterase (AChE) inhibitor, which will stop the destruction of acetylcholine so that it can compete with curare.[42] This can be done by administration of acetylcholinesterase (AChE) inhibitors such as pyridostigmine,[43] neostigmine, physostigmine, and edrophonium. Acetylcholinesterase is an enzyme used to break down the acetylcholine (ACh) neurotransmitter left over in motor neuron synapses. The aforementioned inhibitors, termed "anticurare" drugs, reversibly bind to the enzyme's active site, prohibiting its ability to bind to its original target, ACh. By blocking ACh degradation, AChE inhibitors can effectively raise the amount of ACh present in the neuromuscular junction. The accumulated ACh will then correct for the effect of the curare by activating the receptors not blocked by toxin at a higher rate, restoring activity to the motor neurons and bodily movement.
Gallery
-
Abuta selloana. Certain species in the menispermaceous genus Abuta—particularly the Colombian species A. imene—have sometimes been used in the preparation of curare.
-
Anomospermum schomburgkii. Certain species in the genus Anomospermum have been used in the preparation of some forms of curare.
-
Cissampelos pareira. Certain species in the genus Cissampelos have been employed in the preparation of curare.
See also
- Arrow poison, what curare was originally used for
- Poison dart frog, another source of arrow poison
- Strychnine, a related alkaloid poison that occurs in some of the same plants as curare
References
- ↑ "curare (n.)". Online Etymology Dictionary. Douglas Harper. http://www.etymonline.com/index.php?term=curare.
- ↑ 2.0 2.1 2.2 Manske, R. H. F., ed (1955). The Alkaloids: Chemistry and Physiology – Volume 5, Pharmacology. New York, New York: Academic Press Inc.. p. 269. ISBN 9781483221922. https://books.google.com/books?id=DvbJCgAAQBAJ&pg=PA269. Retrieved 12 May 2014.
- ↑ 3.0 3.1 3.2 3.3 Gibson, Arthur C.. "Curare, a South American Arrow Poison". Plants and Civilization. UCLA Mildred E. Mathias Botanical Garden, University of California, Los Angeles. http://www.botgard.ucla.edu/html/botanytextbooks/economicbotany/Curare/.
- ↑ La Oficina del Indice Histórico de Puerto Rico [The Office of the Historical Index of Puerto Rico] (1949) (in es). Tesauro de datos historicos: Indice compendioso de la literatura histórica de Puerto Rico, incluyendo algunos datos inéditos, periodísticos y cartográficos, Tomo II. San Juan, Puerto Rico: El Gobierno de Puerto Rico. p. 306. https://books.google.com/books?id=IVRnAAAAMAAJ&pg=PA306. Retrieved 4 January 2020.
- ↑ Lee, MR (2005). "Curare: The South American Arrow Poison". The Journal of the Royal College of Physicians of Edinburgh 35 (1): 83–92. PMID 15825249. https://www.rcpe.ac.uk/sites/default/files/curare.pdf.
- ↑ Carman, J. A. (October 1968). "History of curare". Anaesthesia (Association of Anaesthetists) 23 (4): 706–707. doi:10.1111/j.1365-2044.1968.tb00142.x. PMID 4877723.
- ↑ The Gale Encyclopedia of Science (Third ed.). Gale Group.
- ↑ Humboldt, Alexander von; Bonpland, Aimé (1907). Personal Narrative of Travels to the Equinoctial Regions of America, During the Year 1799–1804 – Volume 2. London: George Bell & Sons. https://www.gutenberg.org/ebooks/7014.
- ↑ Brodie, Benjamin Collins (1811). "X. Experiments and Observations on the different Modes in which Death is produced by certain vegetable Poisons. By B. C. Brodie, Esq. F. R. S. Communicated by the Society for promoting the Knowledge of Animal Chemistry". Philosophical Transactions (The Royal Society) 101: 178–208. doi:10.1098/rstl.1811.0011.
- ↑ Brodie, Benjamin Collins (1812). "XI. Further Experiments and Observations on the Action of Poisons on the Animal System. By B. C. Brodie, Esq. F. R. S. Communicated to the Society for the Improvement of Animal Chemistry, and by them to the Royal Society". Philosophical Transactions (The Royal Society) 102: 205–227. doi:10.1098/rstl.1812.0013.
- ↑ "CURARE (Chondrodendron tomentosum - Menispermaceae): From Arrow Poison to Surgical Muscle Relaxant". Ye Olde Log. n.d.. http://www.yeoldelog.com/medicinal/curare.shtml.
- ↑ Waterton, Charles (1891). "Chapter II". Wanderings in South America. London, Paris & Melbourne: Cassell & Company, Limited., reprinted in "Classical File". Survey of Anesthesiology 22 (1): 98 ff. February 1978. https://journals.lww.com/surveyanesthesiology/Citation/1978/02000/WANDERINGS_IN_SOUTH_AMERICA.65.aspx.
- ↑ Birmingham, A T (1999). "Waterton and Wouralia". British Journal of Pharmacology (The British Pharmacological Society) 126 (8): 1685–1689. doi:10.1038/sj.bjp.0702409. PMID 10372809.
- ↑ Paton, A. (December 1979). "George Harley (1829-1896)". Practitioner 223 (1338): 849–51. PMID 396529.
- ↑ "George Harley". Whonamedit? – A dictionary of medical eponyms. http://www.whonamedit.com/doctor.cfm/3230.html.
- ↑ Gray, TC (1947). "The Use of D-Tubocurarine Chloride in Anæsthesia". Ann R Coll Surg Engl (The Royal College of Surgeons of England) 1 (4): 191–203. PMID 19309828.
- ↑ Bernard, Claude (1857). "Vingt-cinquième Leçon [Twenty-fifth Lesson"] (in fr). Leçons sur les effets des substances toxiques et médicamenteuses. Paris: J.B. Baillière. pp. 369–80. https://archive.org/stream/leonssurlesef00bern#page/369/mode/1up.
- ↑ Dale, H. H. (1 November 1914). "THE ACTION OF CERTAIN ESTERS AND ETHERS OF CHOLINE, AND THEIR RELATION TO MUSCARINE". Journal of Pharmacology and Experimental Therapeutics (The American Society for Pharmacology and Experimental Therapeutics) 6: 147–190. http://jpet.aspetjournals.org/content/6/2/147.
- ↑ Dale, Henry (12 May 1934). "Chemical Transmission of the Effects of Nerve Impulses". British Medical Journal 1 (3827): 835–841. doi:10.1136/bmj.1.3827.835. PMID 20778253.
- ↑ King, H. (1935). "Curare alkaloids: Part 1, Tubocurarine". Journal of the Chemical Society (The Royal Society of Chemistry) 57: 1381–1389. doi:10.1039/jr9350001381. https://pubs.rsc.org/en/content/articlelanding/1935/jr/jr9350001381.
- ↑ King, Harold (1935). "Curare". Nature (The Physical Society) 135 (3412): 469–470. doi:10.1038/135469b0. Bibcode: 1935Natur.135..469K. https://www.nature.com/articles/135469b0.
- ↑ 22.0 22.1 Eger, Edmond I II; Saidman, Lawrence J.; Westhorpe, Rod N., eds (2014). The Wondrous Story of Anaesthesia. Springer. p. 438. ISBN 978-1-4614-8440-0. https://books.google.com/books?id=H--3BAAAQBAJ&pg=PA438.
- ↑ ((Editors of Encyclopaedia Britannica)) (7 March 2016). "Curare, chemical compound". Encyclopædia Britannica. https://www.britannica.com/science/curare. Retrieved 17 April 2020.
- ↑ 24.0 24.1 "Curare". Drugs.com. 8 November 2001. https://www.drugs.com/mmx/curare.html.
- ↑ "Curare (Chondrodendron tomentosum - Menispermaceae): From Arrow Poison to Surgical Muscle Relaxant". Ye Olde Log. n.d.. http://www.yeoldelog.com/medicinal/curare.shtml.
- ↑ Schaffner, Brynn (2000). "Curare". Blue Planet Biomes. http://www.blueplanetbiomes.org/curare.htm.
- ↑ Milner, Daniel (Summer 2009). "From the Rainforests of South America to The Operating Room: A History of Curare". Faculty of Medicine, Department of Innovation in Medical Education. University of Ottawa. http://www.med.uottawa.ca/historyofmedicine/hetenyi/milner.html.
- ↑ Lawen, A. (1912). "Über die Verbindung der Lokalanästhesie mit der Narkose, über hohe Extraduralanaesthesie und epidurale injektionen anasthesierender Losungen bei tabischen Makenkrisen" (in de). Beiträge zur klinischen Chirurgie 80: 168–189.
- ↑ Bennett, A. E. (1940). "Preventing traumatic complications in convulsive shock therapy by curare". Journal of the American Medical Association (American Medical Association) 114 (4): 322–324. doi:10.1001/jama.1940.02810040032009.
- ↑ Dennett, Daniel C. (1978). Brainstorms: Philosophical Essays on Mind and Psychology. Cambridge, Massachusetts: The MIT Press. p. 209.
- ↑ Mashraqui, S. (October 1994). "Hypotension induced with d-tubocurarine and halothane for surgery of patent ductus arteriosus". Indian Journal of Anaesthesia 42 (5): 346–50.
- ↑ Beecher, H. K.; Todd, D. P. (1954). "A Study of the Deaths Associated with Anesthesia and Surgery: Based on a Study of 599,548 Anesthesias in Ten Institutions 1948–1952, Inclusive". Annals of Surgery 140 (2): 2–35. doi:10.1097/00000658-195407000-00001. PMID 13159140., reprinted in "Classical File". Survey of Anesthesiology 15 (5): 496 ff. October 1971. doi:10.1097/00132586-197110000-00013.
- ↑ Albertson, HA; Trout, HH; Morfin, E (June 1956). "The Safety of Curare in Anesthesia". Annals of Surgery 143 (6): 833–837. doi:10.1097/00000658-195606000-00012. PMID 13327828.
- ↑ Lewis, Walter H.; Elvin-Lewis, Memory P.F. (1977). Medical Botany: Plants Affecting Man's Health. Wiley-Interscience. ISBN 0-471-53320-3.
- ↑ Kemp, Christopher (17 January 2018). "The Amazonian arrow poison that made modern anaesthesia: Adventurer Richard Gill sought relief from symptoms of multiple sclerosis in an Ecuadorian tribal weapon – with wider results that live on in medicine today". New Scientist (3161). https://www.newscientist.com/article/mg23731610-600-the-amazonian-arrow-poison-that-made-modern-anaesthesia/.
- ↑ Idress, A.H.; Gabrielli, A. (2007). "Techniques of ventilation during CPR". in Paradis, Norman A.; Halperin, Henry R.; Kern, Karl B. et al.. Cardiac Arrest: The Science and Practice of Resuscitation Medicine (2nd ed.). Cambridge, UK: Cambridge University Press. p. 520. ISBN 978-0-521-84700-1. https://books.google.com/books?id=inud-udaRFwC&pg=PA520.
- ↑ McEvoy, Mike (October 12, 2010), Oxymoron: Our Love-Hate Relationship with Oxygen, Albany, New York: Albany Medical College, http://www.dshs.state.tx.us/emstraumasystems/Conference10Handouts/McEvoy_Oxymoron.pdf
- ↑ Damasio, Antonio R. (1999). The Feeling of What Happens: Body and Emotion in the Making of Consciousness. San Diego: Harcourt Brace. p. 357. ISBN 978-0-15-601075-7. https://books.google.com/books?id=RSOPDHP9QekC&pg=PA357.
- ↑ For therapeutic dose of tubocurarine by shorter limit as given in: Rang, H. P. (2003). Pharmacology. Edinburgh: Churchill Livingstone. p. 151. ISBN 978-0-443-07145-4. OCLC 51622037.
- ↑ For 20-fold paralytic dose of toxiferine ("calabash curare"), according to: The Alkaloids: v. 1: A Review of Chemical Literature (Specialist Periodical Reports). Cambridge, England: Royal Society of Chemistry. 1971. p. 330. ISBN 978-0-85186-257-6. https://books.google.com/books?id=R8BlpSvvSfwC&pg=PA330.
- ↑ Cardiopulmonary Resuscitation (CPR), The Gale Group, Inc., 2008, http://medical-dictionary.thefreedictionary.com/cardiopulmonary+resuscitation+(CPR)
- ↑ Saladin, Kenneth S. (2015). Anatomy and Physiology The Unity of Form and Function (7th ed.). New York: McGraw Hill Education. ISBN 978-1259385513.
- ↑ Morgan, Thomas III; Kalman, Bernadette (2007). Neuroimmunology in Clinical Practice. Wiley-Blackwell. p. 153. ISBN 978-1-4051-5840-4. https://books.google.com/books?id=mG4iTVLaCPcC&pg=PA153.
Further reading
- Foldes, F. F. (1993), "Anästhesie vor und nach Curare" (in de), Anaesthesiol Reanim 18 (5): pp. 128–131, PMID 8280340, http://www.general-anaesthesia.com/curare.html, retrieved June 20, 2005
- "Harold Griffith, Fonds P090". Archival Collections Catalogue. Osler Library of the History of Medicine, McGill University Library, McGill University. https://archivalcollections.library.mcgill.ca/index.php/harold-griffith-fonds. – contains papers and records pertaining to Griffith's introduction of curare into anesthesiology
- James, Mel, "Harold Griffith", Canada Heirloom Series, Volume 6, http://collections.ic.gc.ca/heirloom_series/volume6/204-205.htm, retrieved June 20, 2005
- Raghavendra, Thandla (July 2002). "Neuromuscular blocking drugs: discovery and development". Journal of the Royal Society of Medicine 95 (7): 363–367. doi:10.1177/014107680209500713. PMID 12091515.
- Smith, Roger P., "Cholernergic Transmission", Dartmouth College (Trustees of Dartmouth College), http://www.dartmouth.edu/~rpsmith/Cholinergic_Transmission.html, retrieved March 13, 2007
- Strecker, G.J.; Jackson, M.B. (October 1989). "Curare binding and the curare-induced subconductance state of the acetylcholine receptor channel". Biophysical Journal 56 (4): 795–806. doi:10.1016/S0006-3495(89)82726-2. PMID 2479422. Bibcode: 1989BpJ....56..795S.
- Waterton, Charles. Bullen, A. H.. ed. Wanderings In South America. http://infomotions.com/etexts/gutenberg/dirs/etext05/7wnsa10.htm.
 |



