Chemistry:Bobbitt's salt
From HandWiki
Short description: Chemical compound
| |
| Names | |
|---|---|
| IUPAC name
N-(2,2,6,6-tetramethyl-1-oxopiperidin-1-ium-4-yl)acetamide;tetrafluoroborate
| |
| Other names
4-(Acetylamino)-2,2,6,6-tetramethyl-1-oxo-piperidinium tetrafluoroborate
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChemSpider | |
PubChem CID
|
|
| |
| |
| Properties | |
| C11H21BF4N2O2 | |
| Molar mass | 300.10 g·mol−1 |
| Hazards | |
| GHS pictograms | 
|
| GHS Signal word | Warning |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+352, P304+340, P305+351+338, P312, P321, P332+313, P337+313, P362, P403+233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
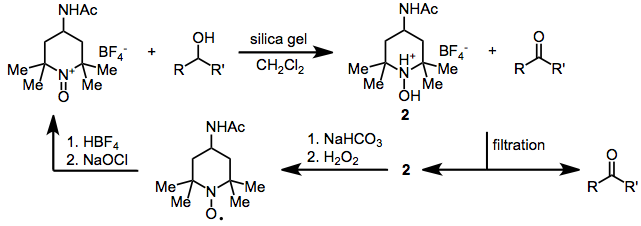
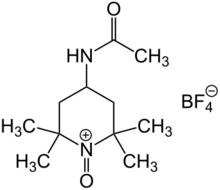
Bobbitt's salt is an oxoammonium compound derived from 4-acetamido-2,2,6,6-tetramethylpiperidine. It contains the tetrafluoroborate anion and is named after the American chemist James M. Bobbitt.
As a less expensive analogue of the N-oxoammonium salt derived from TEMPO, Bobbitt's salt is still mainly used as a catalyst for oxoammonium-catalyzed oxidations.[1][2]
References
- ↑ Nabyl Merbouh; James M. Bobbitt; Christian Brückner (2004). "Preparation of Tetramethylpiperdine-1-oxoammonlum Salts and Their Use as Oxidants in Organic Chemistry. A Review". Organic Preparations and Procedures International 36: 1-31. doi:10.1080/00304940409355369.
- ↑ James M.Bobbitt; Nicholas A.Eddy; Jay J.Richardson; Stephanie A.Murray; Leon J.Tilley (2013). "Discussion Addendum for: Preparation of 4-Acetylamino-2, 2, 6, 6-tetramethylpiperidine-1-oxoammonium Tetrafluoroborate and the Oxidation of Geraniol to Geranial (2,6-Octadienal, 3,7-dimethyl-, (2e)-)". Org. Synth. 90: 215. doi:10.15227/orgsyn.090.0215.
External links
 |