Chemistry:Boldine
| |
| Names | |
|---|---|
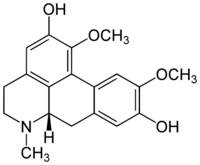
| IUPAC name
1,10-Dimethoxyaporphine-2,9-diol
| |
| Systematic IUPAC name
(6aS)-1,10-Dimethoxy-6-methyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,g]quinoline-2,9-diol | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C19H21NO4 | |
| Molar mass | 327.380 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Boldine is an alkaloid of the aporphine class that can be found in the boldo tree. It is the most abundant aporphine alkaloid found in Boldo.[1] Boldine is also found in Lindera aggregata.[2]
Pharmacology
Boldine has been investigated for its cyto-protective, anti-tumour promoting, anti-inflammatory, anti-diabetic and anti-atherogenic effect and is particularly believed to be a strong antioxidant.[3][4]
Boldine has shown nootropic activity in mice, specifically by significantly improving learning and memory. [5]
Boldine has shown antiepileptic activity in mice against seizures induced by Pentylenetetrazole (PTZ). [6]
Boldine protects against paracetamol induced liver inflammation and acute hepatic necrosis in mice. [7]
Boldine is a 5-HT3 receptor antagonist.[8] 5-HT3A antagonists have been shown to help prevent nausea and vomiting as well as the negative effects of serotonin in the G.I tract. [9][10]
Glaucine is structurally related to Boldine. Glaucine is a dimethyl ether analog of Boldine.
Topical use of DiAcetyl-Boldine in the form of a microemulsion has been studied for chemoprotection against Melanoma.[11]
References
- ↑ O'Brien, P.; Carrasco-Pozo, C.; Speisky, H. (2006). "Boldine and its Antioxidant or Health-Promoting Properties". Chemico-Biological Interactions 159 (1): 1–17. doi:10.1016/j.cbi.2005.09.002. PMID 16221469.
- ↑ Han, Z.; Zheng, Y.; Chen, N.; Luan, L.; Zhou, C.; Gan, L.; Wu, Y. (2008). "Simultaneous Determination of Four Alkaloids in Lindera aggregata by Ultra-High-Pressure Liquid Chromatography–Tandem Mass Spectrometry". Journal of Chromatography A 1212 (1–2): 76–81. doi:10.1016/j.chroma.2008.10.017. PMID 18951552.
- ↑ o'Brien, Peter; Carrasco-Pozo, Catalina; Speisky, Hernán (2006). "Boldine and its antioxidant or health-promoting properties". Chemico-Biological Interactions 159 (1): 1–17. doi:10.1016/j.cbi.2005.09.002. PMID 16221469. https://www.sciencedirect.com/science/article/abs/pii/S0009279705002206.
- ↑ Lau, Yeh Siiang; Ling, Wei Chih; Murugan, Dharmani; Mustafa, Mohd Rais (2015). "Boldine Ameliorates Vascular Oxidative Stress and Endothelial Dysfunction". Journal of Cardiovascular Pharmacology 65 (6): 522–531. doi:10.1097/fjc.0000000000000185. PMID 25469805. PMC 4461386. https://doi.org/10.1097/fjc.0000000000000185.
- ↑ Dhingra, Dinesh; Soni, Kapil (2018). "Behavioral and biochemical evidences for nootropic activity of boldine in young and aged mice". Biomedicine & Pharmacotherapy 97: 895–904. doi:10.1016/j.biopha.2017.11.011. PMID 29136766. https://www.sciencedirect.com/science/article/abs/pii/S0753332217331293.
- ↑ Moezi, Leila; Yahosseini, Siranoush; Jamshidzadeh, Akram; Dastgheib, Mona; Pirsalami, Fateme (2018). "Sub-chronic boldine treatment exerts anticonvulsant effects in mice". Neurological Research 40 (2): 146–152. doi:10.1080/01616412.2017.1402500. PMID 29157166. https://www.tandfonline.com/doi/abs/10.1080/01616412.2017.1402500.
- ↑ Ezhilarasan, Devaraj; Raghunandhakumar, Subramanian (2021). "Boldine treatment protects acetaminophen‐induced liver inflammation and acute hepatic necrosis in mice". Journal of Biochemical and Molecular Toxicology 35 (4): e22697. doi:10.1002/jbt.22697. PMID 33393705. https://onlinelibrary.wiley.com/doi/10.1002/jbt.22697.
- ↑ Walstab, J.; Wohlfarth, C.; Hovius, R.; Schmitteckert, S.; Röth, R.; Lasitschka, F.; Wink, M.; Bönisch, H. et al. (2014). "Natural compounds boldine and menthol are antagonists of human 5-HT3receptors: Implications for treating gastrointestinal disorders". Neurogastroenterology & Motility 26 (6): 810–820. doi:10.1111/nmo.12334. PMID 24708203. https://onlinelibrary.wiley.com/doi/10.1111/nmo.12334.
- ↑ Theriot, J.; Wermuth, H. R.; Ashurst, J. V. (2023). "Antiemetic Serotonin-5-HT3 Receptor Blockers". StatPearls. https://www.ncbi.nlm.nih.gov/books/NBK513318/.
- ↑ "List of 5HT3 receptor antagonists (5hydroxytryptamine receptor antagonists)". https://www.drugs.com/drug-class/5ht3-receptor-antagonists.html.
- ↑ Al Saqr, Ahmed; Annaji, Manjusha; Poudel, Ishwor; Aldawsari, Mohammed F.; Alrbyawi, Hamad; Mita, Nur; Dhanasekaran, Muralikrishnan; Boddu, Sai H. S. et al. (2023). "Topical Delivery of Diacetyl Boldine in a Microemulsion Formulation for Chemoprotection against Melanoma". Pharmaceutics 15 (3): 901. doi:10.3390/pharmaceutics15030901. PMID 36986762.
 |

