Chemistry:Bond dipole moment
The bond dipole moment[1] uses the idea of electric dipole moment to measure the polarity of a chemical bond within a molecule. It occurs whenever there is a separation of positive and negative charges.
The bond dipole μ is given by:
- .
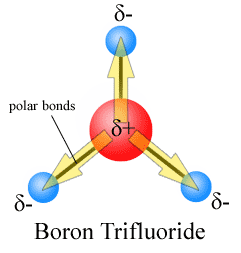
The bond dipole is modeled as δ+ — δ– with a distance d between the partial charges δ+ and δ–. It is a vector, parallel to the bond axis, pointing from minus to plus,[2] as is conventional for electric dipole moment vectors.
Chemists often draw the vector pointing from plus to minus.[3] This vector can be physically interpreted as the movement undergone by electrons when the two atoms are placed a distance d apart and allowed to interact, the electrons will move from their free state positions to be localised more around the more electronegative atom.
The SI unit for electric dipole moment is the coulomb–meter. This is too large to be practical on the molecular scale. Bond dipole moments are commonly measured in debyes, represented by the symbol D, which is obtained by measuring the charge in units of 10−10 statcoulomb and the distance d in Angstroms. Based on the conversion factor of 10−10 statcoulomb being 0.208 units of elementary charge, so 1.0 debye results from an electron and a proton separated by 0.208 Å. A useful conversion factor is 1 D = 3.335 64×10−30 C m.[4]
For diatomic molecules there is only one (single or multiple) bond so the bond dipole moment is the molecular dipole moment, with typical values in the range of 0 to 11 D. At one extreme, a symmetrical molecule such as chlorine, Cl2, has zero dipole moment, while near the other extreme, gas phase potassium bromide, KBr, which is highly ionic, has a dipole moment of 10.5 D.[5][page needed][6][verification needed]
For polyatomic molecules, there is more than one bond. The total molecular dipole moment may be approximated as the vector sum of the individual bond dipole moments. Often bond dipoles are obtained by the reverse process: a known total dipole of a molecule can be decomposed into bond dipoles. This is done to transfer bond dipole moments to molecules that have the same bonds, but for which the total dipole moment is not yet known. The vector sum of the transferred bond dipoles gives an estimate for the total (unknown) dipole of the molecule.
See also
References
- ↑ Blaber, Mike (2018). "Dipole_Moments". California State University. https://chem.libretexts.org/Textbook_Maps/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/Atomic_and_Molecular_Properties/Dipole_Moments.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "electric dipole moment, p". doi:10.1351/goldbook.E01929
- ↑ Hovick, James W.; Poler, J. C. (2005). "Misconceptions in Sign Conventions: Flipping the Electric Dipole Moment". J. Chem. Educ. 82 (6): 889. doi:10.1021/ed082p889.
- ↑ Atkins, Peter; de Paula, Julio (2006). Physical Chemistry (8th ed.). W.H. Freeman. p. 620 (and inside front cover). ISBN 0-7167-8759-8. https://archive.org/details/atkinsphysicalch00pwat/page/620.
- ↑ Physical chemistry 2nd Edition (1966) G.M. Barrow McGraw Hill
- ↑ Van Wachem, R.; De Leeuw, F. H.; Dymanus, A. (1967). "Dipole Moments of KF and KBr Measured by the Molecular‐Beam Electric‐Resonance Method". J. Chem. Phys. 47 (7): 2256. doi:10.1063/1.1703301.
