Chemistry:Brilliant Blue FCF
| |
| |
| Names | |
|---|---|
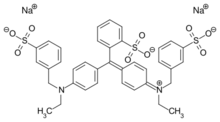
| IUPAC name
disodium;2-[[4-[ethyl-[(3-sulfonatophenyl)methyl]amino]phenyl]-[4-[ethyl-[(3-sulfonatophenyl)methyl]azaniumylidene]cyclohexa-2,5-dien-1-ylidene]methyl]benzenesulfonate
| |
| Other names
FD&C Blue No.1
Acid Blue 9 D&C Blue No. 4 Alzen Food Blue No. 1 Atracid Blue FG Blue #1 Lake Erioglaucine Eriosky blue Patent Blue AR Xylene Blue VSG C.I. 42090, Basacid Blue 755, Sulfacid Brilliant Blue 5 J, Neolan Blue E-A Brilliant Blue FD | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C37H34N2Na2O9S3 | |
| Molar mass | 792.85 g/mol |
| soluble | |
| Hazards | |
| NFPA 704 (fire diamond) | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Brilliant Blue FCF (Blue 1) is a synthetic organic compound used primarily as a blue colorant for processed foods, medications, dietary supplements, and cosmetics.[1] It is classified as a triarylmethane dye and is known under various names, such as FD&C Blue No. 1 or Acid Blue 9. It is denoted by E number E133 and has a color index of 42090. It has the appearance of a blue powder and is soluble in water and glycerol,[2] with a maximum absorption at about 628 nanometers. It is one of the oldest FDA-approved color additives and is generally considered nontoxic and safe.[3]
Production
Brilliant Blue FCF is synthetic dye produced by the condensation of 2-formylbenzenesulfonic acid and the appropriate aniline followed by oxidation.[4] It can be combined with tartrazine (E102) to produce various shades of green.
It is usually a disodium salt. The diammonium salt has CAS number . Calcium and potassium salts are also permitted. It can also appear as an aluminum lake. The chemical formation is C37H34N2Na2O9S3.
Related dyes are C.I. acid green 3 (CAS#4680-78-8) and acid green 9 (CAS#4857-81-2). In these dyes, the 2-sulfonic acid group is replaced by H and Cl, respectively.[5]
Many attempts have been made to find similarly colored natural dyes that are as stable as Brilliant Blue FCF. Blue pigments must possess many chemical traits, including pi-bond conjugation, aromatic rings, heteroatoms and heteroatom groups, and ionic charges in order to absorb low energy red light. Most natural blue dyes are either unstable, blue only in alkaline conditions, or toxic; good candidates for further research into use as natural dyes include anthocyanin and trichotomine derivatives. No replacement for Brilliant Blue FCF has been found for use in beverages.[6]
Applications
Like many other color additives, the primary use of Blue No. 1 is to correct or enhance natural coloring or to give colorless compounds a vivid hue.[7]
In the United States, of the two approved blue dyes (the other being Indigo carmine, or FD&C Blue #2), Brilliant Blue FCF is the more common of the two. As a blue color, Brilliant Blue FCF is often found in cotton candy, ice cream, canned processed peas, packet soups, bottled food colorings, icings, ice pops, blueberry flavored products, children's medications, dairy products, sweets[8] soft drinks, and drinks, especially the liqueur Blue Curaçao. It is also used in soaps, shampoos, mouthwash[9] and other hygiene and cosmetics applications.
Brilliant Blue FCF is extensively used as a water tracer agent.[10] Due to its ability to retain color for long periods of time, Brilliant Blue FCF outperforms other dye tracers. Additionally, Brilliant Blue FCF has a low toxicity level that is favorable for the environment. However, Brilliant Blue FCF has different impacts on varying soils. Brilliant Blue FCF is attracted to and sorbed in acidic soils due to its large size and ionic charge. Soil composition and flow velocity also affect the level of sorption of Brilliant Blue FCF.[11]
Brilliant Blue FCF dye within beverages items—such as soda—can be used in the blue bottle experiment. In such foods, both the dye and reducing agents are incorporated in the same solution. When the solution is blue, oxygen is present. On the addition of NaOH, a reaction occurs that removes the oxygen, turning the solution clear. The dye turns back to blue once it is reoxidized by swirling the solution, incorporating oxygen from the air as an oxidizing agent.[12]
Health and safety
The dye is poorly absorbed from the gastrointestinal tract and 95% of the ingested dye can be found in the feces.
When applied to the tongue or shaved skin, Brilliant Blue FCF can be absorbed directly into the bloodstream.[13]
Due to its nontoxic properties, Brilliant Blue FCF has been used as a biological stain. When dissolved in an acidic medium, this dye has been used to stain cell walls, bacteria, and fungal cells. The dye does not inhibit the growth of any of these species.[14]
For similar reasons, Brilliant Blue FCF is also being utilized in hemostatic medical devices, most notably the HEMOPATCH—designed to be placed on bleeding tissues and coagulate the blood. A low concentration of Brilliant Blue FCF is placed on the backside of the HEMOPATCH at 1 cm increments, allowing surgeons to cut precisely and indicate the side of the HEMOPATCH that is an active hemostatic agent for correct placement.[15]
Brilliant Blue FCF is an approved food colorant and pharmacologically inactive substance for drug formulations in the EU and the United States. It is also legal in other countries. It has the capacity for inducing allergic reactions in individuals with pre-existing moderate asthma.[16] In 2003, the U.S. FDA issued a public health advisory to warn health care providers of the potential toxicity of this synthetic dye in enteral feeding solutions.[17] The following legal limits apply in the EU (E 131) and other countries: 150–300 mg/kg depending on the type of food. Safety limit for foods and drugs: 0.1 mg/day per kg body weight.[18] The ADI for Brilliant Blue FCF is 6 mg/kg.
Biomedical research
Brilliant Blue FCF and similar dyes such as Brilliant Blue G are inhibitors to purinergic receptors—receptors that are responsible for inflammatory responses and other cell process.[19]
Scientists who were conducting in-vivo studies of compounds to lessen the severity of inflammation following experimental spinal cord injury had previously tested a compound called OxATP to block a key ATP receptor in spinal neurons. However, OxATP has toxic side effects and must be injected directly into the spinal cord; in searching for alternatives they noted that Brilliant Blue FCF has a similar structure. This led them to test a related dye, Brilliant Blue G (also known as Coomassie Brilliant Blue) in rats, which improved recovery from spinal cord injury while temporarily turning them blue.[20]
When human washed platelets are evaluated using turbidimetry it was found that Brilliant Blue FCF affects platelet aggregation by blocking the Panx1 channels. These inhibitory effects on collagen-induced shape change and maximal aggregation were shown by high (1 mM) concentrations of the dye but not by lower concentrations (100 μM). The 1 mM effective concentration is 1.59 times greater than the approximately 0.63 mM maximal allowable Brilliant Blue FCF concentration according to the European Food Safety Authority.[21]
Scientists are performing studies to better understand the effects of Brilliant Blue FCF during vein graft explantation. Brilliant Blue FCF hinders the purinergic receptors, limiting cell proliferation that may lead to intimal hyperplasia. The effects of Brilliant Blue FCF were tested on rat aortic cells. It was found that Brilliant Blue FCF had a positive impact in limiting the development of intimal hyperplasia following a vein graft procedure.[22][23]
References
- ↑ "FD&C Blue 1 (Brilliant Blue)". https://www.iacmcolor.org/safety-of-color/safety-synthetic-certified-colors/blue-1/.
- ↑ "FD&C Blue No. 1: Brilliant Blue FCF Food Dye". 2015-08-04. https://culinarylore.com/ingredients:fdc-blue-no-1-brilliant-blue-fcf-food-dye/.
- ↑ Nutrition, Center for Food Safety and Applied (2019-03-16). "Color Additives History". https://www.fda.gov/industry/color-additives/color-additives-history.
- ↑ El Ali, Bassam M.; Bassam El Ali; Ali, Mohammad Farahat (2005). Handbook of industrial chemistry: organic chemicals. New York: McGraw-Hill. ISBN 978-0-07-141037-3. https://archive.org/details/isbn_9780071410373.
- ↑ Gessner, Thomas; Mayer, Udo (2002). "Triarylmethane and Diarylmethane Dyes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a27_179. ISBN 3527306730.
- ↑ Newsome, Andrew G.; Culver, Catherine A.; van Breemen, Richard B. (2014-07-16). "Nature's Palette: The Search for Natural Blue Colorants". Journal of Agricultural and Food Chemistry 62 (28): 6498–6511. doi:10.1021/jf501419q. ISSN 0021-8561. PMID 24930897.
- ↑ Nutrition, Center for Food Safety and Applied (2019-04-14). "Overview of Food Ingredients, Additives & Colors" (in en). FDA. https://www.fda.gov/food/food-ingredients-packaging/overview-food-ingredients-additives-colors.
- ↑ Nestle Aero packet ingredients listing barcode: 7613031579334
- ↑ "LISTERINE Antiseptic Mouthwash, Smart Rinse, Whitening, Advanced, Fluoride Rinse, and Tartar Protection Products". Listerine.com. http://www.listerine.com/product-acb.jsp.
- ↑ "Brilliant Blue FCF as a Dye Tracer for Solute Transport Studies—A Toxicological Overview". Journal of Environmental Quality 23 (5): 1108–1112. 1994. doi:10.2134/jeq1994.00472425002300050037x.
- ↑ "Quantifying preferential flow in soils: A review of different techniques". Journal of Hydrology 378 (1): 179–204. 2009-11-15. doi:10.1016/j.jhydrol.2009.08.013. Bibcode: 2009JHyd..378..179A.
- ↑ "Variations on the "Blue-Bottle" Demonstration Using Food Items That Contain FD&C Blue #1". Journal of Chemical Education 92 (10): 1684–1686. 2015-10-13. doi:10.1021/acs.jchemed.5b00190. Bibcode: 2015JChEd..92.1684S.
- ↑ "Absorption of triphenylmethane dyes Brilliant Blue and Patent Blue through intact skin, shaven skin and lingual mucosa from daily life products". Food and Chemical Toxicology 52: 19–27. February 2013. doi:10.1016/j.fct.2012.10.027. PMID 23127598. "porcine tongue dorsum was exposed to human saliva with 15,000 ng/cm2 of dye for 20 min. 24-h diffusion resulted in 34 ng/cm2 of BB and 86 ng/cm2 of PB which can be directly absorbed into the blood system.".
- ↑ "An innovative brilliant blue FCF method for fluorescent staining of fungi and bacteria". Biotechnic & Histochemistry 86 (4): 280–7. August 2011. doi:10.3109/10520295.2010.492733. PMID 20560873.
- ↑ "Control of bleeding in surgical procedures: critical appraisal of HEMOPATCH (Sealing Hemostat)" (in en). Medical Devices: Evidence and Research 9: 1–10. 2015-12-22. doi:10.2147/mder.s90591. PMID 26730213.
- ↑ "Incidence of bronchoconstriction due to aspirin, azo dyes, non-azo dyes, and preservatives in a population of perennial asthmatics". The Journal of Allergy and Clinical Immunology 64 (1): 32–7. July 1979. doi:10.1016/0091-6749(79)90080-0. PMID 447949. https://www.jacionline.org/article/0091-6749(79)90080-0/pdf.
- ↑ "FD&C Blue No. 1.". Drugs.com. https://www.drugs.com/inactive/fd-c-blue-no-1-244.html.
- ↑ "E133". zusatzstoffe-online.de. http://www.zusatzstoffe-online.de/zusatzstoffe/20.e133_brillantblau_fcf.html.
- ↑ "Brilliant Blue Dyes in Daily Food: How Could Purinergic System Be Affected?". International Journal of Food Science 2016: 7548498. 2016. doi:10.1155/2016/7548498. PMID 27833914.
- ↑ "Systemic administration of an antagonist of the ATP-sensitive receptor P2X7 improves recovery after spinal cord injury". Proceedings of the National Academy of Sciences of the United States of America 106 (30): 12489–93. July 2009. doi:10.1073/pnas.0902531106. PMID 19666625.
- ↑ "Turbidimetry on Human Washed Platelets: The Effect of the Pannexin1-inhibitor Brilliant Blue FCF on Collagen-induced Aggregation". Journal of Visualized Experiments (122). April 2017. doi:10.3791/55525. PMID 28448011.
- ↑ "Use of Brilliant Blue FCF during vein graft preparation inhibits intimal hyperplasia". Journal of Vascular Surgery 64 (2): 471–478. August 2016. doi:10.1016/j.jvs.2015.02.028. PMID 27763268.
- ↑ Voskresensky, Igor V.; Wise, Eric S.; Hocking, Kyle M.; Li, Fan Dong; Osgood, Michael J.; Komalavilas, Padmini; Brophy, Colleen; Cheung-Flynn, Joyce (2014-11-01). "Brilliant Blue FCF as an Alternative Dye for Saphenous Vein Graft Marking: Effect on Conduit Function". JAMA Surgery 149 (11): 1176–81. doi:10.1001/jamasurg.2014.2029. ISSN 2168-6254. PMID 25251505.
Further reading
- "Chronic toxicity of two food colors, brilliant blue FCF and indigotine". Toxicology and Applied Pharmacology 8 (1): 29–36. January 1966. doi:10.1016/0041-008X(66)90097-4. PMID 5950860.
- "Lifetime toxicity/carcinogenicity studies of FD & C Blue No. 1 (brilliant blue FCF) in rats and mice". Food and Chemical Toxicology 28 (4): 221–34. April 1990. doi:10.1016/0278-6915(90)90034-K. PMID 2358248.
- "Synthesis of 14C-labelled FD & C Blue No. 1 (Brilliant Blue FCF) and its intestinal absorption and metabolic fate in rats". Food and Cosmetics Toxicology 18 (1): 1–5. February 1980. doi:10.1016/0015-6264(80)90002-4. PMID 7372204.
External links
- The Chemistry of Colors
- Some more details, other common names
- FDA Public Health Advisory on use in enteral feeding
- The Feingold Diet; Dubious Benefits, Subtle Risks





