Chemistry:Calcium morphenate
From HandWiki
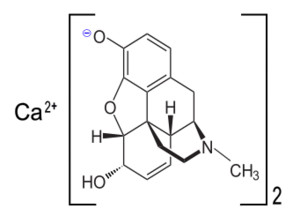
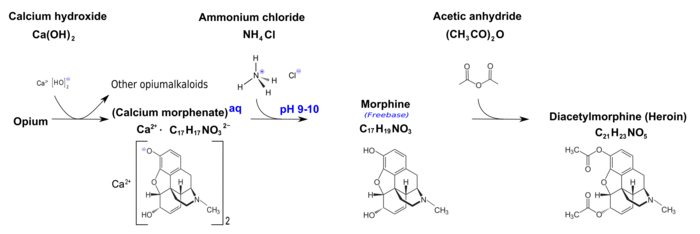
Calcium morphenate is a calcium salt of morphine which is produced by using calcium bases to raise the pH of an aqueous solution of opium alkaloids to around 9.0.[1] This was a method used in pharmaceutical manufacturing to separate morphine from other alkaloids and inert materials from the opium solution. Variations on this route are still used in Afghanistan.[2] When poppy straw concentrate or opium latex is dissolved in hot water and the calcium base is added, calcium morphenate is formed. Treatment with a weak acid such as ammonium chloride then causes morphine freebase to precipitate, leaving codeine and other alkaloids of the plant in solution.
References
- ↑ "Method of Extraction of Morphine and Related Derivatives" US patent 2062324
- ↑ U. Zerell, B. Ahrens and P. Gerz (2005). "Documentation of a heroin manufacturing process in Afghanistan". Bulletin on Narcotics LVII (1 and 2). https://www.unodc.org/pdf/research/Bulletin07/bulletin_on_narcotics_2007_Zerell.pdf.
 |
