Chemistry:Calyculin
From HandWiki
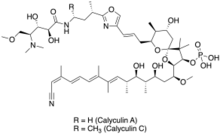 Calyculins A and C
| |
| Names | |
|---|---|
| IUPAC name
((2R,3R,5R,7R,8S,9S)-2-((1S,3S,4S,5R,6R,7E,9E,11E,13Z)-14-cyano-3,5-dihydroxy-1-methoxy-4,6,8,9,13-pentamethyltetradeca-7,9,11,13-tetraenyl)-9-((E)-3-(2-((2S)-4-(((2S,3S,4S)-4-(dimethylamino)-2,3-dihydroxy-5-methoxypentanoyl)amino)butan-2-yl)-1,3-oxazol-4-yl)prop-2-enyl)-7-hydroxy-4,4,8-trimethyl-1,10-dioxaspiro(4.5)decan-3-yl) dihydrogen phosphate (Calyculin A)[1]
| |
| Identifiers | |
| |
| ChEMBL | |
| ChemSpider |
|
PubChem CID
|
|
| UNII |
|
| |
| Properties | |
| C51H83N4O15P (Calyculin C) | |
| Molar mass | 1023.2 g/mol (Calyculin C) |
| Properties | |
| C50H81N4O15P (Calyculin A) | |
| Molar mass | 1009.17 g/mol (Calyculin A) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Calyculins are natural products originally isolated from the marine sponge Discodermia calyx.[2] Calyculins have proven to be strong serine/threonine protein phosphatase inhibitors and based on this property, calyculins might be potential tumor-promoting agents.
References
- ↑ "Calyculin A". 2015-01-02. https://media.cellsignal.com/pdf/9902.pdf.
- ↑ "Calyculin A, an inhibitor of protein phosphatases, a potent tumor promoter on CD-1 mouse skin". Cancer Res. 50 (12): 3521–3525. 1990. PMID 2160320.
 |
- ↑ Tanimoto, Norihiko; Gerritz, Samuel W.; Sawabe, Akiyoshi; Noda, Takeshi; Filla, Sandra A.; Masamune, Satoru (1994-03-17). "Synthese von natürlich vorkommendem (−)‐Calyculin A" (in en). Angewandte Chemie 106 (6): 674–677. doi:10.1002/ange.19941060611. ISSN 0044-8249. https://onlinelibrary.wiley.com/doi/10.1002/ange.19941060611.
- ↑ [1]

